About Authors: Kollu Varuni, B.pharm
Vathsalya college of pharmacy,
J.N.T.U University
Introduction:
Tamsulosin is a selective, potent and competitive a 1 – adrenoreceptor antagonist and has a greater affinity for these receptors, predominantly present in the human prostate. Literature survey reveals that several methods like HPLC,HPLC-MS and LC-MS were reported for the estimation of Tamsulosin hydrochloride in combination with other drugs as well as in biological fluids [1- 3]
Tamsulosin is extensively metabolized by CYP-450 in the liver.
CHEMISTRY OF TAMSULOSIN HCL
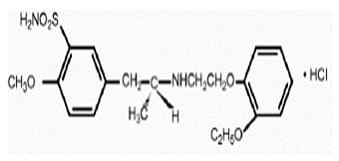
Molecular formulae : C20H28N2O5S •HCl.
Molecular weight : 444.98 g/mol
Melting Point : melts with decomposition at approximately 230°C.
Chemical name : (-)-(R)-5-[2-[[2-(o-Ethoxyphenoxy) ethyl]amino]propyl]-2-methoxybenzenesulfonamide, monohydrochloride.
Physical properties: white crystalline powder
Solubility characters: sparingly soluble in water and methanol, slightly soluble in glacial acetic acid and ethanol, and practically insoluble in ether.
[adsense:336x280:8701650588]
USES
1.Tamsulosin HCl a selective alpha blocker used for the treatment of benign prostatic hyperplasia (enlarged prostate) in men.
2. sometimes used for the passage of kidney stones by the same mechanism of smooth muscle relaxation via alpha antagonism.
Adverse Reactions
CNS
Headache (21%); dizziness (17%); somnolence (4%); decreased libido (2%); insomnia (1%).
ENT
Rhinitis (18%); pharyngitis (5%); amblyopia (2%).
GI
Diarrhea (6%); nausea (4%); tooth disorder (2%).
Genitourinary
Abnormal ejaculation (18%).
Respiratory
Increased cough (5%); sinusitis (4%).
Miscellaneous
Infection (11%); asthenia (9%); back pain (8%); chest pain (4%).
Analytical chemistry may be derived as the science and art of determining the composition of material in terms of the elements of compounds contained. By means of analytical techniques both qualitative analysis (the presence or absence of one or more elements) and quantitative analysis (how much amount is present) can be done.
The qualitative and quantitative analysis can be done by various analytical methods: various analytical techniques can be revised and some of them give accurate result
METHODS OF ANALYSIS:
Generally analytical methods are classified into:
(a) Chemical methods
(b) Instrumental methods
CHEMICAL METHODS:
(1) Volumetric methods
(2) Gravimetric methods
INSTRUMENTATION METHODS
(1) Electrochemical methods
Electrogravimetric method ,Colorimetry, Potentiometry, Conductometry, Polarography.
(2) Optical methods
Emission spectroscopy, Luminescence analysis, X-ray spectroscopy, Raman spectroscopy, Atomic absorption spectroscopy, Absorption spectroscopy, Turbidimetry, Nephelometry, Refractometry.
(3) Radiometric methods
Isotopic dilution
(4) Chromatographic Techniques
HPLC - High performance Liquid Chromatography
GC - Gas Chromatography
(5) Mass spectroscopy
(6) Nuclear Magnetic Resonance
PROPERTIES USED FOR MEASUREMENT
(1) Mass
(2) Volume
(3) Electrical properties
(4) Absorption of radiation
(5) Emission of radiation
(6) Scattering of radiation
(7) Rotation of radiation
(8) Diffraction of radiation
(9) Mass of charge ratio
(10) Thermal properties
STEPS IN QUANTITATIVE ANALYSIS
(1) Sampling: Sampling involves selecting a representative sample of the material to be analysed. The sample should be reasonably pure and should be homogeneous.
(2) Conversions of desired constituents to measurable form: This involves the method of separation. The selection of separation techniques for a specific situation depends on a number of factors. Such selection is generally decided on the basis of accuracy and the precision required.
(3) Measurement of desired constituents: Any physical or chemical property can be used as a mean or qualitative identification and quantitative measurement or both if the property is specific and selective for measurement. Then the separation and pretreatment of the sample can be minimized. The measurement step in an analysis can be carried out by chemical, physical or biological mean.
(4) Calculation and interpretation of analytical data: An analysis is not complete until the results have been expressed in such a manner that the person, for whom the results intended, can understand their significance.
SELECTION OF A METHOD FOR ANALYSIS
The basic requirements are repeatability and reproducibility.
Repeatability
It means agreement or closeness of results amongst duplicate or triplicate obtained by a single analyst under a given set of conditions. The same analyst working in the same laboratory should get duplicate or triplicate results with in expected limits of variation.
Reproducibility
The extent of agreements obtained when the same test is carried out by different analysts of different laboratories. Thus reproducibility is a more characteristic feature of a given method than repeatability. The following factors should be considered before selecting analytical method:
(1) Simplicity: By simplicity, we mean that the analysis can be carried out in a minimum number of steps and need only easily available reagents and apparatus. More number of steps involved, more chances of personal errors creeping in the method must be easy to adopt by small laboratories with limited resources of equipments and semi skilled personnel.
(2) Ruggedness: A Method which could be adopted by all laboratories is called “rugged”. In other words expected small variation from person to person, laboratory to laboratory, atmosphere to atmosphere will not affect the precision of a method. The adoption of a rugged method facilitates quality assurance method.
(3) Specificity, Selectivity and Interference: When carrying out a particular test in the prescribed way the analyst assumes that the results of the test reference only to the component being tested and are not affected by the presence of other component for instance Barium chloride test for sulphates are not affected by the presence of other constituents. In such cases we say that the method is specific. For instance the EDTA titration method is not specific for calcium, other elements such as zinc and magnesium also gives the same reaction it is called “selective” but can be made specificity by choosing an indicator which responds only for calcium. Even specific and selective tests could give inaccurate values if there is interference from other components.
(4) Sensitivity: It refers to the smallest quantity or magnitude of a particular constituent which can be quantitatively measured with a precision equal to that claimed for the determination as whole sensitivity means the minimum amount of the component that can be measured under stated conditions. For instance a visual colorimetric method for iron may just differentiate between two samples containing 30 – 35 ppm. We then say the method is sensitive to 5 ppm.
(5) Precision and Accuracy: The relative of a given method from various types of errors can be broadly designed as either “precision or accuracy” end use of the product will dictate the extent of accuracy. If a method is very precise but not accurate the correct result can still be obtained by a method of calibration.
(6) Range of application: It indicates the range of concentration which would be detected by a method. For example any titration method is applicable only for higher concentration while the instrumental method is generally good only for lower concentration.
(7) Toxicity and Hazards: Chemicals as solvent and reagents excellent methods are ruled out because of toxicity and hazards. For example use of poisonous chemicals as solvents and reagents
(8) Time cycle: Time taken to complete analysis more steps involve more time to complete. For industrial consideration the time cycle is very important.
(9) Cost of Analysis: It is significant as far as industries are concerned. The simplicity of the method the number of steps involved the time cycle the availability and the cost of reagents, the requirement of costly instruments and skilled and experienced analysts are major contributors.
AIM OF WORK: Although various methods have been developed for the estimation of Tamsulosin HCl by HPLC method, as per literature review the methods like UV-spectrophotometry, HPLC, HPTLC and LC-MS/MS for the estimation these drug individually and in combination with other drugs. But I am trying to develope new estimation method of this drug by HPLC method individually.
PLAN OF WORK
An attempt is made in this project to device a simple cost effective, accurate, sophisticated, precise and sensitive method like HPLC for Tamsulosin HCL.
It includes the following
• Method development
• Validation
• Assay
HIGH PERFORMANCE LIQUID CHROMATOGRAPHY (HPLC)
HPLC was introduced commercially in 1969 and since then it has undergone extensive modifications and innovation which lead to its emergence as the foremost analytical tool for quantitative analysis. HPLCis a type of liquid chromatography that employs a liquid mobile phase and a very finely divided stationary phase. In order to obtain a satisfactory flow rate liquid must be pressurized to a few thousands of pounds per square inch.
The rate of distribution of drugs between stationary and mobile phase is controlled by diffusion process. If diffusion is minimized, a faster and effective separation can be achieved. The technique of high performance liquid chromatography is so called because of its improved performance when compared to classical column chromatography. Advances in column technology, high pressure pumping system and sensitive detectors have transformed liquid column chromatography into high speed, efficient, accurate and highly resolved method of separation.
For the present study, the drug Tamsulosin HCl was selected for their estimation. The HPLC method was considered the choice of estimation, since this method is the most powerful of all chromatographic and other separative methods. The HPLC method has enabled analytical chemist to attain great success in solving his analytical problems.
The HPLC is the method of choice in the field of analytical chemistry, since this method is precise, accurate and linear and also it offers the following advantages.
- Speed (many analysis can be accomplished in 20 minutes or less).
- Greater sensitivity (various detectors can be employed).
- Improved resolution (wide variety of stationary phases)
- Reusable columns (expensive columns but can be used for many analysis).
- Ideal for the substances of low volatility.
- Easy sample recovery, handling and maintenance.
- Instrumentation leads itself to automation and quantitation (less time and less labour)
- Precise and reproducible.
- Calculations are done by integrator itself.
- Suitable for preparative liquid chromatography on a much large scale.
Types of HPLC
The type of HPLC methods include:
• Normal phase chromatography
• Reversed phase chromatography.
Normal Phase Chromatography
The term normal phase refers to a system where a stationary phase is polar and mobile phase is a relatively non polar liquid (hexane, benzene, CHCL?, etc). In this mode most probably used stationary phase is silica gel. The silica structure is saturated with silanol groups at the end and ‘OH’ groups attached to silicon atoms are the active binding sites. Best separating compounds include plasticizer, dyes, steroids, amines, alkaloids, alcohols, phenols, aromatic and metal complexes.
Reversed Phase Chromatography
A most popular mode for analytical and preparative method for separation of compounds of interest in chemical, biological, pharmaceutical, food and biomedical sciences. In this mode the stationary phase is a non polar hydrophobic packing with octyl or octadecyl functional group bonded to the silica gel and mobile phase is a polar solvent. An aqueous mobile phase allows the use of secondary solute chemical equilibria (such as ionization control, ion suppression, ion pairing and complexation) to control retention and selectivity.
The polar compound gets eluted first in this mode and non-polar compounds are retained for a larger time. Since most of the drugs and pharmaceuticals are polar in nature, they are not retained for longer time and eluted faster. The different columns used are octadecyl silane (ODS) or C18, C8, C4 etc (In order to increase the polarity of the stationary phase). Hence by varying the organic moiety in the silianization reagent (dimethyl chlorosilane derivatives) different stationary phase of polarities can be realized4,5.
Importance of Polarity in HPLC: The relative distribution of solute between two phases is determined by the interactions of the solute species with each phase. In both normal phase and reversed phase HPLC, the eluting power or solvent strength of the mobile phase is mainly determined by its polarity. The relative strengths of these interactions are determined by the polarity of the sample and the mobile and stationary phase.
Reverse Phase Mobile Phases
The power of HPLC in terms of being able to resolve many compounds is mainly due to the diversity of mobile phases or mobile solvents available. The mobile phase in HPLC, however, has a great influence on the retention of the solutes and the separation of component mixtures.
The primary constituent of reversed phase-mobile phase is water. Water miscible solvents such as methanol, ethanol, acetonitrile, dioxane, tetrahydrofuran and dimethyl formamide are added to adjust the polarity of the mobile phase. They should be of high quality, either distilled or demineralised. The most widely used organic modifiers are methanol, acetonitrile and tetrahydrofuran. Methanol and acetonitrile have comparable polarities but the latter is an aprotic solvent. This factor may be important if hydrogen bonding plays a significant role in the separation. When inorganic salts and ionic surfactants are used, the mobile phase should be filtered before use since these additives frequently contain a significant amount of water-insoluble contaminants that may damage the column. Degassing is quite important with reverse-phase mobile phases1.
Polarity is a term that is used in chromatography as an index of the ability of compounds to interact with one another. It is applied very freely to solute, stationary and mobile phase. If the polarities of stationary phase and the mobile phase are similar, it is likely that the interactions of solute with each phase may also be similar, resulting in poor separation. Retention of solutes is usually altered by changing the polarity of the mobile phase. Successful chromatographic separation requires a proper balance of intermolecular force among three participants in separation process i.e Analyte, Mobile phase and Stationary phase2.
Methods of Quantitative Analysis in HPLC
The sample or solute is analysed quantitatively in HPLC by either peak height or peak area measurements. Peak areas are proportional to the amount of constant rate. Peak heights are proportional to the amount of material only when peak width are constant and are strongly affected by the sample injection techniques. Once the peak height or the peak areas are measured, there are five principle evaluation methods for quantifying the solute3.
a) Calibration by Standards
Calibration curves for each component are prepared from pure standards, using identical injection volumes of operating conditions for standards and samples. The concentration of solute is read from its curve if the curve is linear.
X = K x area
Where,X = Concentration of solute
K = Proportionality constant (slope of the curve)
In this evaluation method only the area of the peaks of interest is measured. Relative response factors must be considered when converting areas to volume and when the response of a given detector differs for each molecular type of compounds.
b) Internal Standard Method
In this technique a known quantity of the internal standard is chromatographed and area vs. concentration is ascertained. Then a quantity of the internal standard is added to the raw sample prior to any sample pretreatment or separation operations.
The peak area of the standard in the sample run is compared with the peak area when the standard is run separately. This ratio serves as a correction factor for variation in sample size, for losses in any preliminary pretreatment operations, or for incomplete elution of the sample. The material selected for the internal standard must be completely resolved from adjacent sample components, must not interfere with the sample components and must never be present in samples.
Area of sample
Area ratio = -----------------------------------
Area of internal standard
Area ratio of sample
Sample concentration = ----------------------------------
Area ratio of standard
c) Area Normalization
The technique is often used for the sample having identical components. It is used to evaluate the absolute purity of the sample. The procedure is to total up the areas under all peaks and then calculate the percentage of the total area that is contributed by the compound of interest. For this method the entire sample must be eluted, all components must be separated and each peak must be completely resolved.
d) Standard Addition Method
If only few samples are to be chromatographed, it is possible to employ the method of standard addition(s). The chromatogram of the unknown is recorded, then a known amount of analyte(s) is added and the chromatogram is repeated using same reagents, instruments and other conditions. From the increase in the peak area (or peak height), the original concentration can be computed by interpolation.
The detector response must be a linear function of analyte concentration and yield no signal at zero concentration of the analyte. Sufficient time must elapse between addition of the standard and actual analysis to allow equilibrium of added standard with any matrix interferant.
If an instrumental reading (area/height) ‘Rx’ is obtained, from a sample of unknown ‘x’ and a reading ‘Rt’ is obtained from the sample to which a known concentration ‘a’ of analyte has been added, then ‘x’ can be calculated from:
X Rx
-------- = ----------
x+a Rt
A correction for dilution must be made if the amount of standard added changes the total sample volume significantly. It is always advisable to check the result by adding at least one other standard.
e) External Standard Method
It employs a separate injection of a fixed volume of sample and standard solution. The peaks are integrated and concentration is calculated.
Peak area of sample
Sample concentration = ------------------------------- x Conc. Of Standard
Peak area of standard
The selection of suitable chromatographic (HPLC) system for a given mixtures of solutes cannot be made with certainty and must be confirmed by experiment.
If the chemical nature of the sample components is known, then the phase system can be selected from the literature references.
If nothing is known about the chemical nature of sample, then the sample solubility will give some indication as to which chromatographic method to employ.
The essential parts of high performance liquid chromatographic system are solvent reservoir, pump, injection port, column, detector and recorders2.
Solvent reservoir
Stainless steel and glass are used for making solvent reservoir. They should be inert to a variety of aqueous and non-aqueous mobile phases. Stainless steel should be avoided for solvents containing halide ions. If the reservoir is to be pressurized, glass is to be avoided. The capacity of the reservoir should be greater than 500ml. The aqueous and organic solvents are degassed prior to use in order to prevent the formation of gas bubbles in the detector. Degassing is done by following methods.
Ø By stirring the mobile phase in vacuum
Ø Purging with helium gas and
Ø Ultrasonication
Ø Finally the solvent is filtered through Millipore filter before introducing into the reservoir.
Pumps
the pumps must be constructed from materials that are inert to all mobile phases. Materials used are stainless steel, glass and Teflon. They should generate a pressure up to 800 psi at a flow rate of up to 3ml/minute and should provide pulse less solvent flow. The solvent should be dampened to remove pulses, since the presence of pulses in solvent flow may cause spurious results with some detectors. The pump should produce reproducible and constant flow rate HPLC pumps can be classified into two groups according to the manner in which they operate. They are constant flow rate pumps and constant pressure pump. The two principle types of constant flow rate pump are reciprocating piston pump and syringe dive pump.
Reciprocating pump has filling and pumping cycle. During the filling cycle a piston is withdrawn from a syringe type chamber. Two check-valves are connected to this chamber such that during the piston withdrawal, solvent flows from the reservoir to the pump outlet. The volume of solvent discharged from the pump in unit time can be changed by altering the distance that the piston travels (or) the number of cycles. The advantage of this pump is unlimited volume of the solvent reservoir since it is external to the pump.
Syringe drive pump is a single stroke displacement pump in which all of the mobile phase is contained within the pump. The piston inside the chamber is actuated by a screw feed drive connected to a stepping motor. The volume displaced by the pump per unit time is controlled by the voltage applied. This pump produces a pulse less flow and requires no check valves.
Constant pressure pump can deliver a constant flow rate if the pump operates against a constant column back pressure and if the viscosity of the mobile phase remaining constant. Constant pressure pump may be simple gas displacement pump (or) pneumatic amplifier pump simple gas displacement pump is a reservoir such as coil of tubing to which pressure is applied from a gas cylinder. The disadvantage of this pump is limited solvent capacity. Pneumatic amplifier pump is a modification of simple gas displacement pump. The gas pressure is applied to a large piston, which is connected to a small diameter piston, which is in contact with mobile phase when all the mobile phase is used up, the piston returns quickly by pneumatic means, thus refilling the chamber. The advantage of this pump is pulse less flow and unlimited solvent capacity.
Sample injection system
The injection of sample on to the column presents some unique problems because of high pressure involved in HPLC. The volume of sample used ranges from 0 to 500 μl. The various injection methods are
Syringe Injection
The sample is injected by two method using micro syringes, which are designed to with stand pressure up to 1500 psi. The injection is done through a self-sealing elastomeric septum.
Stop Flow Injection
The type of syringe injection can be made as high pressure but not through the septum. Here the flow of the solvent is momentarily stopped and the sample is directly injected on to the head of the column. This method is simple and convenient.
LoopInjection (or) Sampling Valve
This system is the most widely used injection system, which provides precise injection volumes against high back pressures. In this method a sample loop of fixed capacity is connected to a high-pressure valve and the sample is filled onto the sample loop through a syringe.
Column
HPLC column are made up of stainless steel or glass, which differs in length and inside diameter depending on the application. The two types of columns are analytical column and preparative column. Standard analytical columns are 4-5mm in internal diameter and 10-30 cm in length. The particle size used ranges from 5-10 micrometers. Preparative columns are 20-50 mm in internal diameter and 20-100 cm in length. The particle size used ranges from 37-50 micrometers.
Detector
All detectors used in HPLC may be of selective type and non-selective type in which the former detectors only a part of the components and the later detects almost all the components. The choice of the detector depends on the mobile phase, nature of the analyte and the required sensitivity. The detectors used are Refractive index detector, U.V. absorption detector, fluorescence detector, electrochemical detector, mass detector and radioactive detector.
Recorder
The signals from the detector are recorded as deviation from a baseline. Two open recorders are used with instruments having two detectors. The peak position along the curve relative to the starting point, denotes the particular component, with proper calibration the height or area of peak is a measure of the amount of the component present in the sample.
Best column, best mobile phase, best detection wavelength, efforts in their selection can make a world of difference while development HPLC method for routine analysis. Determining the ideal combination of these factors assures faster delivery of desired results a validated method for separation3.
ANALYTICAL PARAMETERS USED IN ASSAY VALIDATION
Accuracy: Often expressed as percentage recovery by the assay of known added amounts of analyte.
Precision: A measure of either the degree of reproducibility or repeatability of the analytical method under normal operating condition.
Linearity and range: The linearity of an analytical method is its ability to elict test the linearity of results that are directly proportional to the concentration of analyte in sample in a range
REVIEW OF LITERATURE
* L. R. Snyder, J.J. Kirkland, and J. L. Glajch, Practical HPLC Method Development, John Wiley & Sons, New York, 1997.
* Hassan Y Aboul – Enein, Rajaa F Hussein, Mahasen A Radwan, Ahmad Yusuf, Wijdan Al Ahmadi and Sameer Al Rawithi, Tamsulosin dissolution from pharmaceutical dosage forms using an automated HPLC system.J Liq. Chrom & Rel. Tech,26(7), 2003, 1007-1009.
* Macek J, Klama J, and Ptajeek P, Rapid determination of Tamsulosin in human plasma by HPLC using extraction with butyl acetate. J. Chrom B. Anlyt Technol. Biomed Life Science , 809(2), 2004, 307-311.
* Ming Zhao ; Hartke Carol ; Jimeno Antonio ; Jing Li ; Ping He ; Zabelina Yelena ; Hidalgo Manuel (1) ; Baker Sharyn D. Journal of chromatography. B, 2005, vol. 819[1], pp. 73-80.
* Guetens G., Prenen H., De Boeck G., Van Dongen W., Esmans E, Lemiere F., Van Oosterom A. T., Schöffski P., De Bruijn E. A. Journal of chromatography, 2005, vol 1082[1], pp 2-5.
* M.W. Dong, Modern HPLC for practicing scientists. Wiley, 2006.
* S. Ahuja and H. T. Rasmussen (ed), HPLC Method Development for Pharmaceuticals, Academic Press, 2007.
* Keski Rah Konen P, Parssinen O, Leppaonen E, Mauriala T, Lehtonen M and Auriola S, Determination of Tamsulosin in human aqueous humor and Serum by liquid chromatography – electrospray ionization Tandem mass spectrometry. J Pharm Biomed Aval,43(2),2007,606-612. RP-HPLC method for the estimation of tamsulosin hydrochloride in bulk and tablet dosage form- T.S.Nithiyananthan*,V.Shankarananth1, K.K.Rajasekhar1, P.Ravikiran1, E.Vikram kumar1 and G.Jayanth kumar reddy1*,1-Sri Padmavathi School of Pharmacy, Tiruchanoor, Tirupati-517503,Andhra Pradesh,India. 15-07-2009. A simple reverse phase HPLC method for the estimation of Tamsulosin hydrochloride in bulk and tablet dosage form. The estimation was carried out on ODS,Phenomonex,C-18 (250x4.6 mm,5μ) column using a mobile phase consisting of sodium dihydrogen orthophosphate buffer-Acetonitrile (70:30).The eluent was monitored at 280 nm. The results have been validated statistically and recovery studies confirmed the accuracy of proposed method.
* FORMULATION AND EVALUATION OF TAMSULOSIN HYDROCHLORIDE AS SUSTAINED RELEASE MATRIX TABLET- T.S. Nithiyananthan*, V. Shankarananth, K.K. Rajasekhar, G. Hareesh, P. Naveen Kumar & R. Siva Prasada Reddy. International Journal of ChemTech Research CODEN( USA): IJCRGG ISSN : 0974-4290 Vol.1, No.4, pp 1278-1290, Oct-Dec 2009. Prolonged, sustained or extended release systems, release the active ingredient slowly than conventional dosage forms similarly administered. Tamsulosin is a selective, potent and competitive α1 – adrenoreceptor antagonist. It has a greater affinity for the α1A – receptor subtype and is indicated to treat uretheral stone symptoms associated with benign prostatic hyperplasia. In the present work, attempt was made to develop an once daily sustained release matrix tablet of Tamsulosin hydrochloride. Hydroxy propyl methyl cellulose was used as a hydrophilic matrix polymer. The formulation showed acceptable pharmacotechnical properties and HPLC assay requirements. The present work also involves application of Higuchi’s equation, Drug release kinetics, Korsmeyer’s and Hixsen-crowell plots. In vitro drug release studies indicated a sustained release pattern.
* L. R. Snyder, J.J. Kirkland, and J. W. Dolan, Introduction to Modern Liquid Chromatography, John Wiley & Sons, New York, 2009.
* Development and validation of RP-HPLC method for the estimation of tamsulosin hydrochloride in bulk drug & pharmaceutical dosage form Sanjay Dinkar Sawant*, Minal Rushikesh Ghante, Asmita Shripad Deshpande, Neha Manish Munot Sinhgad Technical Education Society’s Smt. Kashibai Navale College of Pharmacy, Kondhwa (Bk), Pune – 411 048. 2010. A rapid, reproducible and selective reverse phase HPLC method has been developed for the estimation of tamsulosin hydrochloride in bulk and capsule dosage form. It was resolved by using a mobile phase of phosphate buffer : acetonitrile: methanol in the ratio 50:40:10 v/v of pH 5.0 at a flow rate of 1 ml/min. on an isocratic HPLC system using UV Visible detector at the wavelength of 275 nm. The column used was ODS C-18 RP column (4.6 mm x 250mm).
PROCEDURE
INSTRUMENT EMPLOYED
Shimadzu HPLC, 10 AT detector, Rheodyne injector with 20µl loop was used, mode of operation – Isocratic and Temperature ambient
HPLC columns -Hypersil ODS C18 Column 250 X 4.6 mm , 5 µ
Inersil C18 column 150 X 4.6 mm ,5 µ
Water Sperisorb column 250 X 4.6 mm ,5 µ
Hypersil BDS C18 Column 250 X 4.6 mm (particle size of 5 µ).
Electronic Balance
Ultra Sonicator
Thermal Oven
pH analyzer
Triple Quartz Distillation Unit
HPLC Injecting Syringe (25 ml) (HAMILTON)
REAGENTS USED
1. Acetonitrile HPLC grade
2. Methanol HPLC grade
3. Water-HPLC grade
4. Phosphoric acid HPLC grade
5. Tamsulosin HCl
TAMSULOSIN HCL
TRAIL METHOD -I
Buffer : Ammonium acetate.
Mobile phase: Buffer : Acetonitrile (75:25)
λ max : 279nm
Flow rate : 0.5 ml/min
Column: Hypersil BDS C8 250 x 4.6 mm 5µm
Retention Time : 14.4 min
TRAIL METHOD –II
Buffer: Sodium dihydrogen phosphate
Mobile phase : Buffer : ACN (60:40)
λ max: 279nm
Flow rate: 0.5 ml/min
Column: Waters Spherisorb ODS 250 x 4.6 mm 5µm
Retention Time: 5.9min
TRAIL METHOD –III
Buffer: Sodium dihydrogen phosphate
Mobile phase: Buffer : ACN (60:40)
λ max: 279nm
Flow rate: 0.5 ml/min
Column: Inertsil 150 x 4.6 mm 5µm
Retention Time: 2.817 min
RESULT and DISCUSSION
From the optical characteristics of these proposed methods, it was found that trail method III for Tamsulosin HCl obey Accaracy, Precision and Linearity within the concentration respectively.
From the results ,it was found that the percentage recovery values of pure drugs from the preanalyzed solution of formulation were 99.23 % for Tamsulosin HCl ,which indicates that the proposed method is accurate and also reveals that the commonly used excipients and additives in the pharmaceutical formulations were not interfering in the proposed method.
CHROMATOGRAMS ARE ATTACHED:
Blank_Tamsulosin Hcl
Spiking Std01_Tamsulosin(120mcg)
Spiking Std02_Tamsulosin(120mcg)
Spiking Std03_Tamsulosin(120mcg)
Spiking Std04_Tamsulosin(120mcg)
Spiking Std05_Tamsulosin(120mcg)
SUMMARY AND CONCLUSION
Pharmaceutical analysis occupies a pivotal role in statuary certification of drugs and their formulations either by the industry or by the regulatory authorities. In industry, the quality assurance and quality control departments play major role in bringing out a safe and effective drug and dosage form. The current good manufacturing practices (cGMP) and the Food and Drug Administration (FDA) guidelines insist for adoption of sound methods of analysis with greater sensitivity and reproducibility. Therefore, the complexity of problems encountered in pharmaceutical analysis with the importance of achieving the selectivity, speed, low cost, simplicity, sensitivity, specificity, precision and accuracy in estimation of drugs.
Although various methods have been developed for the estimation of Tamsulosin HCl individually, the present work done on this comprises a simple, precise and accurate method by reverse phase high performance liquid chromatography.
The present drug of Tamsulosin HCl was marketed as formulation.
Tamsulosin HCl – 0.4 mg/Cap
An attempt has been made to estimate Tamsulosin HCl by RP-HPLC. Even though a number of RP-HPLC methods have been reported earlier for Tamsulosin HCl individually and with other combinations, an effort has been made to identify a common mobile phase to come up with the isocratic elution of drug individually.
The formulation was diluted in the linearity range and peak areas were determined, the concentrations of Tamsulosin HCl were then determined by comparing the peak areas of sample with that of standard peak areas of that can be identified by their retention times for Tamsulosin HCl.
The results obtained from HPLC method were reproducible and encouraging. The values percentage deviation was satisfactorily low and recovery close to 100% indicating reproducibility and accuracy of method.
The proposed method were proved to be superior to most of the reported method and this can be used as alternative method for the routine determination of selected drugs under the study in bulk and pharmaceutical dosage forms.
Thus the purpose of the present investigation was successfully achieved.
REFERENCES
1. Guetens G., Prenen H., De Boeck G., Van Dongen W., Esmans E, Lemiere F., Van Oosterom A. T., Schöffski P., De Bruijn E. A. Journal of chromatography, 2005, vol 1082[1], pp 2-5.
2. L. R. Snyder, J.J. Kirkland, and J. W. Dolan, Introduction to Modern Liquid Chromatography, John Wiley & Sons, New York, 2009.
3. L. R. Snyder, J.J. Kirkland, and J. L. Glajch, Practical HPLC Method Development, John Wiley & Sons, New York, 1997.
4. M.W. Dong, Modern HPLC for practicing scientists. Wiley, 2006
5. Ming Zhao ; Hartke Carol ; Jimeno Antonio ; Jing Li ; Ping He ; Zabelina Yelena ; Hidalgo Manuel (1) ; Baker Sharyn D. Journal of chromatography. B, 2005, vol. 819[1], pp. 73-80.
6. Pao W, Miller V, Zakowski M, et al. (September 2004). "EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to Tamsulosin HCl and erlotinib". Proceedings of the National Academy of Sciences of the United States of America 101 (36): 13306–11. doi:10.1073/pnas.0405220101. PMID 15329413.
7. Sordella R, Bell DW, Haber DA, Settleman J (August 2004). "Tamsulosin HCl-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways". Science (New York, N.Y.) 305 (5687): 1163–7. doi:10.1126/science.1101637. PMID 15284455.
8. S. Ahuja and H. T. Rasmussen (ed), HPLC Method Development for Pharmaceuticals, Academic Press, 2007.
9. Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008
10. V Kiran Kumar, N Appala Raju, Shabana Begum, Jvln Seshagiri Rao and T Satyanarayana ISSN 0974-3618 , 2009, Research J. Pharm. and Tech.2 (2): April.-June.2009; Page 341-343
Reference ID: PHARMATUTOR-ART-1008
FIND OUT MORE ARTICLES AT OUR DATABASE









