 About Authors:
About Authors:
Gunjan Kalyani*, Vishal S. Deshmukh, Pranita Kashyap, Ram D. Bawankar, Yogesh Vaishnav, Deepak Biswas
Shri Rawatpura Sarkar Institute of Pharmacy,
Behind power grid, Kumhari, Durg, Chhattisgarh
*kalyani.gunjan@yahoo.in
Abstract:
A simple, rapid and precise and accurate RP-HPLC method was developed for the estimation of candesartan. The method involves a simple technique using Irbesartan as internal standard. HPLC separation was achieved using C18 Intersil column (256 x 4.6 id) with an isocratic mobile phase composed of selected methanol: 10 mM Phosphate buffer [75:25 % v/v. pH 3.0] at a flow rate of 1.0 mL/min with UV detection was performed at 256 nm. The retention time of candesartan and internal standard was found to be 1.96 and 3.33 min respectively. The assay was validated for the parameters like accuracy, precision, robustness and system suitability parameters. The method was validated over a linear test concentration range of 80 - 120%. The recovery of the method was in between 99.0 - 101.0%. The proposed method was found to be accurate, precise, selective and rapid and it can be useful in the routine analysis for the determination of candesartan cilexetil in pharmaceutical dosage form.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1480
Introduction
Candesartan cilexetil (prodrug is a racemic mixture containing one chiral center at the cyclohexyloxy-carbonyloxy- ethyl ester group. Angiotensin II receptor blockers (ARBs) are a new class of therapeutic agents for hypertension. The ARBs have a more direct mechanism of action than other drugs affecting the angiotensin converting enzyme inhibitors[1]. Candesartan is a potent, highly selective ARB that is devoid of agonist activity. It is administered orally as Candesartan cilexetil, which is rapidly and completely hydrolyzed to Candesartan, the active moiety, during absorption from the gastrointestinal tract. Candesartan cilexitil is white to off white powder. It is soluble in dimethyl formamide, acetone, methanol, 0.1 N sodium hydroxide solution and insoluble in water[1].
[adsense:468x15:2204050025]
Mechanism of action[1]:
Candesartan cilexetil is a prodrug that has little pharmacological activity until it is hydrolyzed to candesartan during absorption. Candesartan competes with angiotensin II for binding at the AT1 receptor subtype. The drug blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland9. Candesartan, ARB, is used alone or with other antihypertensive agents to treat hypertension. The action is different from ACE inhibitors, which block the conversion of angiotensin I to angiotensin II, meaning that the production of angiotensin II is not completely inhibited, as the hormone can be formed via other enzymes. Also, unlike ACE inhibitors, candesartan and other ARBs do not interfere with response to bradykinins and substance P, which allows for the absence of adverse effects that are present in ACE inhibitors (e.g. dry cough). Various chromatographic methods have been published for the determination of candesartan in pharmaceutical dosage forms. Literature survey also revealed various HPLC methods are reported for the estimation of candesartan cilexetil using internal standards such as bumetanide, bromhexine etc. in human plasma. It would therefore be beneficial to provide accurate, precise and reliable methods for determination of candesartan using irbesartan as internal standard. The method involves a simple technique using irbesartan as internal standard. The present work describes analytical procedures for the quantitation of candesartan and irbesartan as internal standard in formulation using RP-HPLC.
Fig 1. Structure of candesartan
Experimental
Chemicals
All reagents used were of HPLC and AR grade purchased from Loba Chemicals, Mumbai, India. Reference standard of candesartan cilexetil and internal standard Irbesartan were obtained from Dr. Reddy’s Laboratories, Hyderabad, India. The candesartan tablets were procured from local market, brand name CANDESAR (4 mg), manufactured by RANBAXY Laboratories, India.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Instrumentation
High performance liquid chromatography:
The LC system consists of Jasco PU 1580 intelligent pump, a Rheodyne injector equipped with a 20 μl sample loop, and a Variable wavelength UV/VIS detector (Jasco UV 1575). The output signal was monitored and integrated using Borwin Software (version 1.21.60).
Chromatographic conditions
C18, Intersil (256 X 4.6 i.d., particle size 10μm); analytical column was used for separation. The mobile phase was delivered through the column at a flow rate of 1.0 ml/min. Column was operated at room temperature. The sample injection volume was 20 μl. The variable wavelength UV/VIS detector was set at a wavelength of 256 nm. The internal standard was added in fixed concentrations.
Mobile phase preparation
The mobile phase consisted of methanol: 10 mM Phosphate buffer [75:25 % v/v] (pH 3.0). The mobile phase was filtered through Whatman filter paper No.41 and degassed by sonication for 20 min. Standard stock solutions of candesartan cilexetil and irbesartan (1 mg/ml) were prepared in mobile phase. To study the linearity range of the drugs, serial dilutions were made from standard stock solution in the range of 0-140 μg/mL of candesartan and irbesartan. A graph was plotted as concentration of drug versus peak area response, and it was found to be linear for both the drugs. From the standard stock solutions, mixed standard solution was prepared containing 10 μg/mL of candesartan cilexetil and irbesartan. The system suitability test was performed collecting data from replicate injections of mixed drug solutions.
Twenty tablets, Candesar were weighed and finely powder and quantity equivalent to 10 mg of candesartan cilexetil was transferred in a 25 ml volumetric flask, along with about 10 mg of internal standard, irbesartan and were dissolved in sufficient quantity of methanol, sonicated for 20 min and volume was made upto the mark with the help of mobile phase. The solution was filtered through Whatman filter paper No.41. The aliquot portion of the filtrate was further diluted to get final concentrations of 10 μg/ml of candesartan cilexetil and irbesartan. Twenty microliters of the test and standard solutions were injected separately and chromatogram was recorded for the same, and the amounts of the drugs were calculated. Linearity and range of method was determined on standard solution by analyzing 80 to 120 % of test concentration, and the calibration curve was plotted using AUC versus concentration of standard solution. Accuracy of method was ascertained by recovery study by adding a known amount of standard drug (± 20 % of test concentration) to preanalysed sample and reanalyzing the samples by proposed method. The average recovery of candesartan cilexetil and irbesartan in the 98 to 100.23 %. Precision was studied by analyzing five replicates of sample solution. Ruggedness of method was evaluated by performing the assay with different analysts and on different days. The chromatographic parameters were also validated by system suitability studies (Table 3), which were carried out on freshly prepared standard stock solutions. The typical chromatogram obtained from the formulation is presented in fig. 2. The retention time for candesartan cilexetil and irbesartan was found to be 1.96 and 3.33 min, respectively. Peaks were well resolved with resolution of 5.44 between the two drugs and were symmetrical in shape with symmetry factor less than 1.20.
Results and discussion
The technique of quantitation involves the use of an internal standard to compensate for various analytical errors. Whatever the manipulations are performed during the method to get final concentration of analyte as well as internal standard; the error will be compensated by treating both simultaneously and therefore accuracy of the method increases. In proposed method mobile phase used consist of methanol and phosphate buffer pH 3.0 which is very easy to prepare and no organic modifier is required for the pH adjustment. The mobile phase, methanol and 10 mM phosphate buffer in ratio of 65:35 % v/v, get adjusted to pH 3 which is less tedious and less time consuming. In proposed method irbesartan is used as internal standard. The method show linear standard calibration curve with coefficient of correlation=0.9999. The proposed method with R.S.D. = 0.0042, indicate that the proposed method is sensitive. The method show good recovery which is within limit i.e. (98 - 100.23%) indicates there is no interference of excipients.
Limit of detection (LOD) and limit of quantitation (LOQ)
The calibration curve was plotted in concentration range of 0 - 140 μg/ml of candesartan cilexetil and the fixed concentration of internal standard irbesartan i.e. 4 μg/ml and it was found to be linear with the correlation coefficient of 0.9999. The LOD and LOQ were separately determined based on the calibration curve. The standard deviation of the Y-intercept and slope of the regression lines were used. Limit of quantification was found to be 0.58 μg/mL and Limit of detection was found to be 0.19 μg/mL.
Conclusion
Proposed method is simple, less tedious, and more economic, less time consuming for the determination of candesartan cilexetil using irbesartan as an internal standard. The proposed method can be used for the routine determination of drug in bulk and pharmaceutical dosage form.
Acknowledgement
The author (Gunjan Kalyani) is thankful to Dr. Reddy’s Laboratories, Hyderabad India for providing free samples of Candesartan cilexetil and irbesartan for the thesis work. Author (Gunjan Kalyani) would sincerely thank Principal, my teacher Vishal S. Deshmukh Sir, Yogesh Vaishnav Sir, and Ajit Pandey Sir, staff & lab technicians SRIP, Kumhari, Durg, C.G. for their contribution in carrying forward the research work, for providing the necessary support, for his kind and valuable guidance, and for providing basic facilities in lab.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
|
S.No. |
Concentration (µg/ml) Candesartan |
Ratio of area Can:IS (Irb) |
|
1 |
5 |
0.5781 |
|
2 |
10 |
1.4347 |
|
3 |
15 |
2.0518 |
|
4 |
30 |
4.1983 |
|
5 |
40 |
5.6661 |
|
6 |
100 |
13.8546 |
|
7 |
120 |
16.7974 |
|
8 |
140 |
19.5221 |
Table 1 Study of linearity
|
Drug |
Label Claim (mg/ml) |
Estimation* |
Recovery** |
||
|
Mg Tablet |
% Label claim |
Amount added in µg/ml |
% Recovery |
||
|
candesartan |
4 |
3.99
|
99.75 |
3.2 |
99.59 (0.76) |
|
4 |
99.92 (0.89) |
||||
|
4.8 |
100.02 (0.67) |
||||
Table 2: Analysis of Marketed formulations and recovery studies
|
Parameters |
Candesartan |
Irbesartan |
|
Retention Time (min) |
1.96 |
3.33 |
|
Tailing Factor |
1.07 |
1.10 |
| Plate no. | 2410.4 | 2834.1 |
| Resolution | 5.44 | |
| Selectivity | 1.99 | |
| LOD | 0.58μg/ml | |
|
LOQ |
0.19μg/ml |
|
Table 3: System suitability Parameters
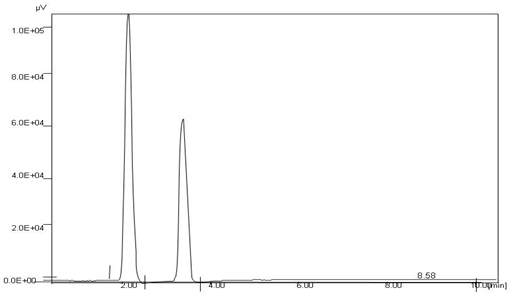
Fig. 2: Chromatograms of Candesartan (Rt = 1.96), Irbesartan (Rt = 3.33) in Marketed formulation
References
1. Ganesh Akula, Kandikonda Saikrishna, RP- HPLC method development and validation of candesartan cilexetil in bulk and their pharmaceutical dosage forms, IJPSR, 2010; Vol. 1 (12): 191-196.
2. United State Pharmacopoeia XXIV NF 19, 21st ed.,2001.
3. The Merck Index,14thed.,Merck and Co. Inc., USA: White House station , NJ, 2007
4. Martindale, The complete drug reference, Pharmaceutical Press, 35th ed., Vol. I, 2007
5. Indian Pharmacopoeia, Govt. of India. Ministry of Health and Family Welfare. Vol. 2. Publication by Indian Pharmacopoeia Commission, Ghaziabad, 200
6. British Pharmacopoeia, International Edition, Vol. II. Published by the Stationary Office on behalf of The Medicine and Healthcare Products Regulatory Agencies (MHRA), London SNQ, 2008.
7. Erk NJ. Liquid chromatography and Related technologies. 2003;26(15):2581-2591
8. Alonso RM, Gonzalez L and Lopez JA. J. Chromatography A. 2002; 949:49- 60.
9. Yokasai IA, Garba M, Musa H and Gwarzo MS. Nigerian J Pharm Research. 2004;3(1):567.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









