{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Nyira Shafi*1, Mohd Akbar Dar1, Mubashir Hussain Masoodi1, Urfan Nabi2
1Department of Pharmaceutical Sciences,
School of Applied Sciences and Technology,
University of Kashmir, Srinagar, J&K, India
2 Department of Pharmacology,
Govt. Medical College Srinagar, J&K, India
ABSTRACT
Purpose: To study the adverse drug reactions (ADR’s) reported from Dermatology ward in SMHS hospital of Srinagar. The adverse drug reactions were analyzed by Naranjo’s causality assessment scale and the outcomes were studied.
Methods: This observational and cross-sectional study was conducted for one month of July 2018 in an inpatient setting of Dermatology ward of SMHS hospital. The data collection was done only in Dermatology ward. Patients of all age groups and either sex were included in this study. The adverse drug reactions were assessed for their causality by performing the Naranjo’s algorithm scale. The outcomes were studied.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2613
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 10 Received On: 19/08/2018; Accepted On: 05/09/2018; Published On: 01/10/2018 How to cite this article: Shafi, N., Dar, M.A., Masoodi, M.H. and Nabi, U. 2018. A Preliminary Study of Adverse Drug Reactions in a Tertiary Care Hospital of Kashmir. PharmaTutor. 6, 10 (Oct. 2018), 37-40. DOI:https://doi.org/10.29161/PT.v6.i10.2018.37 |
Result: Total 3 adverse drug reactions were reported from Dermatology ward. 20-40 years age group was studied for adverse drug reactions. The most commonly affected organ is the Skin 2 (66.66%), followed by Reproductive system 1 (33.34%).The 3 reported ADR’s were Acute Urticaria in 2 patients and Azoospermia in 1 patient. According to Naranjo’s algorithm scale, out of 3 suspected ADR’s 2 (Azoospermia: Cyclophosphamide and Acute Urticaria: Sulfasalazine) were probable and 1 suspected ADR (Acute Urticaria: Tetanus Toxoid) was possible. Out of 3 patients, 2 with Acute Urticaria recovered while as patient with Azoospermia continued with the problem during study.
Limitations: Study was conducted only in one ward i.e., Dermatology and patient number was also less.
INTRODUCTION:
Drugs are intended to use for diagnosis, prevention, treatment and as prophylaxis of various diseases. But it is sometimes observed, that these drugs also show some adverse effects. This could be due to variable person-to-person response towards a drug. Even at therapeutic doses, people develop adverse effects. Adverse effects have been classified in many ways. One may classify them into two categories: predictable (Type A or Augmented) reactions and Unpredictable (Type B or Bizarre) reactions. World health organization (WHO) defines an adverse drug reaction (ADR),” As a response to a drug which is noxious and unintended and which occurs at doses normally used for prophylaxis, diagnosis or therapy of disease or for the modification of physiological function”. According to epidemiological studies, ADR’s account to 3.4% of hospital admissions and 1.8% mortality in India (Charu Bahri, 2016). It is the 4th leading cause of death all over world (Lazarou J, 1998). ADR’s are a leading cause of morbidity and mortality in many countries. It is a great concern for public, medical professionals, pharmaceutical industry, regulatory authorities and other health care professionals. India is a country which has supported the WHO program for global monitoring of ADR’s that is done by spontaneous reporting, because of this reporting system, many drugs were banned in the market for the safety reasons. Government of India launched the pharmacovigilance programme of India (PVPI) in 2010, its main function is to monitor adverse drug reactions by establishing Adverse Drug Reactions Monitoring Centers (AMC) across India. Currently there are 170 AMC in India (Singh KNM, 2017).
REVIEW OF LITERATURE
Effect of cyclophosphamide on spermatozoa is well documented (Jianping Qiu et al (1992), who reported that cyclophosphamide may have an adverse effect on spermatozoa. A study was also conducted on the use of Testosterone to prevent Cyclophosphamide induced Azoospermia. They reported that continuous use of Cyclophosphamide for 6-8 months induces Azoospermia (infertility) (Masala, et al 1997).
In another conducted study on Drug induced urticaria where it was reported that Drug induced Urticaria is most commonly caused by Penicillins, Sulfonamides and NSAIDs (Shipley D, et al., 2001).
Adverse Reactions To Tetanus Toxoid and it is reported there that Urticaria is one of ADR’s of Tetnus toxoid (WG White et al.1973)
Immediate Allergic Reactions after administration of Tetanus toxoid on 4 children. The study showed that children developed urticaria as an ADR (Myorga C, et al. 2003).
OBJECTIVE:
1. To study the adverse drug reactions (ADR’s) reported from Dermatology ward in SMHS hospital of Srinagar.
2. To bring awareness regarding adverse reactions of various drugs.
METHODOLOGY:
This observational and cross-sectional study was conducted for 1 month (July 2018) in an inpatient setting of dermatology ward of SMHS hospital in Srinagar Kashmir. The data collection was done only in one ward. Patients of all age groups and either sex were included in this study. The Adverse drug reaction form filled by health care professionals was considered. The adverse drug reactions were assessed for their causality by performing the Naranjo’s algorithm scale. The outcomes were studied.
Exclusion criteria: OPD patients are excluded. The use of alternative system of medicines such as Ayurveda, Homeopathy, Unani etc, excess consumption, was excluded. Patients who are mentally retarded, drug addicted, suicidal tendencies or consumption of a drug in the influence of alcohol was also excluded.
RESULTS AND DISCUSSION:
In this study only 3 patients were reported to experience an ADR. Total 3 adverse drug reactions were reported from Dermatology ward. 20-40 years age group was studied for adverse drug reactions. The most commonly affected organ is the Skin 2 (66.66%), followed by Reproductive system 1 (33.34%). The 3 reported ADR’s were acute Urticaria in 2 patients and Azoospermia in 1 patient. According to Naranjo’s algorithm scale, out of 3 suspected ADR’s 2 (Azoospermia: Cyclophosphamide and Acute Urticaria: Sulfasalazine) were probable and 1 suspected ADR (acute Urticaria: Tetanus Toxoid) was possible. Out of 3 patients, 2 with Acute Urticaria recovered while as patient with Azoospermia continued with the problem during study (Table 1 & 2).
CASE 1. A 20 year old female patient who received Tetanus Toxoid and experienced Acute Urticaria .Tetanus Toxoid was prescribed to the patient for the treatment of forehead injury. Therapy began on 23rd July 2018 and was withdrawn on the same date.
CASE 2. A 30 year old male patient who received cyclophosphamide and experienced Azoospermia. Cylophosphamide was prescribed to the patient for the treatment of Pemphigus vulgaris. Therapy began on 10 0ctober 2016 and was withdrawn on 7th DCP cycle of therapy 27 September 2017.
CASE 3. A 36 year old female patient who received Sulfasalazine and experienced Acute Urticaria. Sulfasalazine was prescribed to the patient for the treatment of Arthritis. Therapy began on 18 June 2018 and was withdrawn on 24 July 2018.
Table-1: Details of ADR
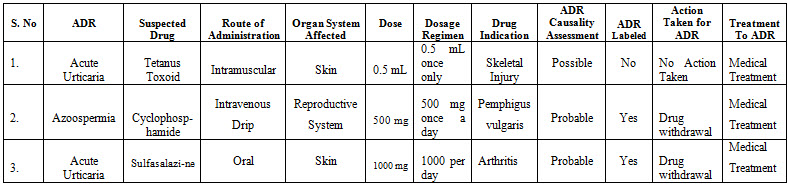
Table-2: Patient details
|
S. No. |
ADR |
Patient's Age |
Sex |
Concomitant Medication |
Relevant Medical History |
|
1. |
Acute Urticaria : Tetanus Toxoid |
20 years |
Female |
No |
History of Tetanus Toxoid Injection 3 times from last 3 months |
|
2. |
Azoospermia : Cyclophosphamide |
30 years |
Male |
Dexamethasone, Calcium Citrate Malate |
Patient a known case of Pemphigus vulgaris from last 3 years |
|
3. |
Acute Urticaria : Sulfasalazine |
36 years |
Female |
Methylprednisolone |
No relevant medical history available |
CONCLUSION:
Adverse drug reaction is an undesirable or unintended consequence of drug administration. It is a broad term, includes all kinds of noxious effects-trivial, serious or even fatal. The main aim of studying adverse drug reactions is to establish a system of rational use of drugs. In this study few cases of ADR were considered from inpatients of dermatology ward. After an ADR, the drug was withdrawn and re-challenge was not performed in any patient. In order to make safety and rational use of drugs there is need of more concern from health care professionals towards ADRs and their reporting.
REFERENCES:
1. A Masala, R Faedda et al (1997); Use of testosterone to prevent cyclophosphamide-induced azoospermia. Annals of Internal Medicine; 126(4); 292-5.
2. Antonio Masala, R Faedda et al., (2017); Use of testosterone to prevent cyclophosphamide-induced azoospermia. Annals of Internal Medicine; 126(4); 292-5.
3. Charu Bahri (2016); How India tackles adverse drug reactions- by ignoring data. India Spend, Doi: 08-02-2016.
4. Jianping QIU, Barba F, Barbaire HB (1995); Damage to Rat Spermatozoal DNA after Chronic Cyclophosphamide Exposure. Biology of Reproduction; 52; 33-40.
5. Lazarou J, Pomeranz B, Corey PN (1998); Incidence of adverse drug reactions in hospitalized patient; JAMA; 279; 1200-1205.
6. Mammen SJ (2018); A Study of Adverse Drug Reactions in a Tertiary care Hospital of Pune; PharmaTutor; 6(8); 38-43.
7. Mayorga C, Torres MJ, Corzo JL, Alvarez J, García JA, Rodríguez CA, Blanca M, Jurado A (2003); Immediate allergy to tetanus toxoid vaccine: determination of immunoglobulin E and immunoglobulin G antibodies to allergenic proteins; Annals of Allergy, Asthma & Immunology; 90(2); 238-43.
8. Shipley D, et al., (2001); Cyclosporine in severe psoriasis. Results of a meta-analysis in 579 patients; American Journal of Clinicallcal Dermatology 2(1):41-47.
9. Singh KNM, Kanase HR (2017); The beginning, current status and recent progress. Adv Pharmacoepidemiol Drug sap; 6; 219
10. White WG, Barnes GM, Barker E, Gall D, Knight P, Griffith AH, Morris-Owen RM, Smith JWG (1973); Reactions to tetanus toxoid; The Journal of Hygiene; 71(2): 283–297.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









