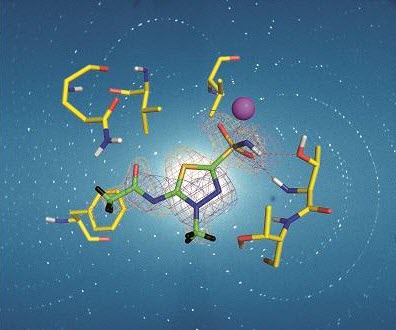
More than 20 years ago, a billboard in China piqued the interest of a chemical biologist. It endorsed an extract from the plant known as the "thunder god vine" as an immunosuppressant. A brief review of published research revealed that the extract's key ingredient, the small molecule triptolide, had been identified 20 years before that billboard ad, and it could stop cells from multiplying.