Capturing Immunotherapy Response in a Blood Drop
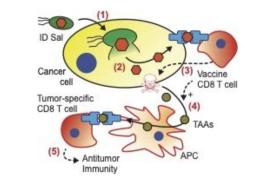
Liquid biopsies are blood tests that can serially measure circulating tumor DNA (cell-free DNA that is shed into the bloodstream by dying cancer cells). When used in patients with advanced non-small cell lung cancer undergoing immunotherapy, they may identify patients who could benefit from treatment with additional drugs, according to a phase 2 clinical trial in the U.S. and Canada.


















.png)


