Alembic Pharmaceuticals’s joint venture, Aleor Dermaceuticals Limited, has received US Food and Drug Administration (USFDA) Tentative Approval for Diclofenac Sodium Topical Solution USP, 2% w/w.

Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
Alembic Pharmaceuticals’s joint venture, Aleor Dermaceuticals Limited, has received US Food and Drug Administration (USFDA) Tentative Approval for Diclofenac Sodium Topical Solution USP, 2% w/w.

Sun Pharma along with its wholly owned subsidiaries is a defendant in a multi-district litigation brought by various classes of plaintiffs, in the US District Court (District of Massachusetts), alleging a delay in the market entry for three generic drugs which are Valganciclovir, Valsartan and Esomeprazole.
Pharmacists, all over the India are opposing Union Health Ministry’s Notification dated 06th November, 2019 regarding proposal to amendment in Schedule K of the Drugs and Cosmetics Rules which is allowing unqualified or lesser qualified workers to dispatch medicines.
About 10% of bone implants lead to failure due to post-surgical bacterial infections. To overcome this hurdle, often heavy dosage of medicines are injected or given orally
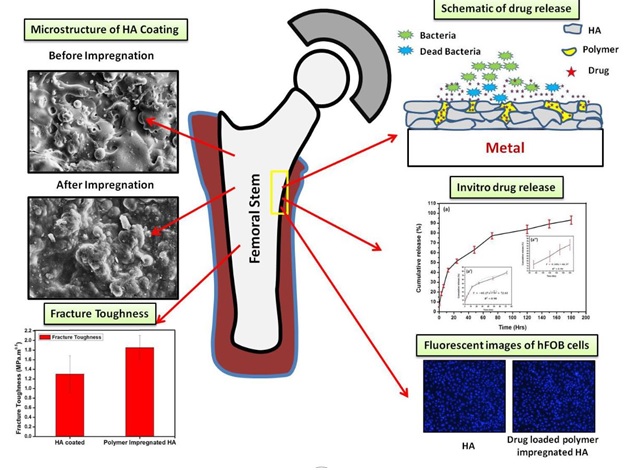
A team of researchers from India and the US has now come up with a method to localise the drug delivery to surgery site.
Indian researchers have identified a protein pathway in an antibiotic-resistant bacterial strain called Staphylococcus aureus (S. aureus) and also a new molecule that can target this pathway.
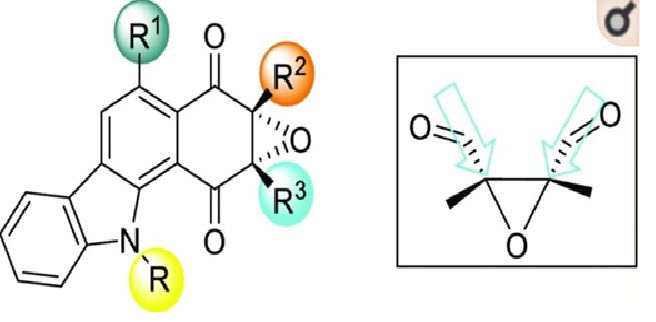
This may help develop new antibacterial drugs in future.
With their expertise in the safe and effective use of medications, pharmacists can help in the management of chronic diseases. A review and analysis published in the British Journal of Clinical Pharmacology indicates that initiatives--such as patient education, medication review, and physical assessments - led by pharmacists can make important contributions to the prevention of cardiovascular disease.
As part of Project Orbis, a collaboration with the Australian Therapeutic Goods Administration (TGA) and Health Canada, the U.S. Food and Drug Administration granted supplemental approval to Calquence (acalabrutinib) for the treatment of adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). This new approved indication for Calquence provides a new treatment option for patients with CLL or SLL as an initial or subsequent therapy.
The Accelerating Medicines Partnership (AMP) program for Parkinson’s disease (PD) has launched a data portal to provide de-identified information collected from 4,298 PD patients and healthy controls to researchers working to develop effective therapies for the disease. The portal enables researchers to study complex data sets and perform genome-wide analyses at a scale previously impossible.
(adsbygoogle = window.adsbygoogle || []).push({});
The “WHO status report on prison health in the WHO European Region” presents an analysis of data collected on the health status of people in prison and prison health systems for 39 countries in the Region. The WHO survey collected data from Member States between 2016 and 2017 to enable monitoring and surveillance of health in prisons.
The U.S. Food and Drug Administration approved XCOPRI (cenobamate tablets) to treat partial-onset seizures in adults.
A seizure is a usually short episode of abnormal electrical activity in the brain. Seizures can cause uncontrolled movements, abnormal thinking or behavior, and abnormal sensations. Movements can be violent, and changes in consciousness can occur. Seizures occur when clusters of nerve cells (neurons) in the brain undergo uncontrolled activation. A partial-onset seizure begins in a limited area of the brain.