Q.1. (c) Explain origin of uv/visible absorption spectrum through it. What information may be sought regarding structure of organic compounds?
Ans.1. (c) Origin of ultraviolet absorption spectra:
[adsense:336x280:8701650588]
Ultra violet absorption spectra arise from transition of electron or electrons with in a molecule or an ion from a lower to a higher electronic energy level. Both organic and inorganic species exhibit electronic translations in which outermost of bonding electrons are promotes to higher energy levels. A compound appears colored if it absorbs light in the visible region and reflects the light of wavelength in the rest of the visible region. The main function of absorbed energy is to raise the molecule from the ground state energy E0 to the higher excited state (energy E1) shown in fig. 1.3.
The difference E1 – E0 = ΔE is given by
ΔE = E1 – E0= hv = h c/λ (v = c/λ)
Where, h = Planck’s constant
c = Velocity of light
λ = wave length of the absorbed radiation.
Δ E depends upon how tightly the electrons are bound in the bonds and accordingly the absorption will occur in UV or particular region of visible range.
The electrons occupy definite orbits, the energy Δ E and the frequency of light absorbed must have definite values. The frequency of the absorbed light is associated with a particular line in the spectrum. The spectrum of the compound will consist of a will consist of a large number of lines corresponding to a large number of excited states of the large number of molecules constituting the compound. The values of Δ E are very close to each other; the lines appear as a band. The existence of the bands in definite parts of the spectrum produces the color.
The total energy of the molecule is the sum of its electronic energy, its vibrational energy and its notational energy. This is due to the fact that there may be three changes in the molecule as a result of absorption of radiation.
(a) There may be rotation of molecule.
(b) There may be vibration of combining atoms, within the molecule.
(c) There may be electronic transition from one orbit to another.
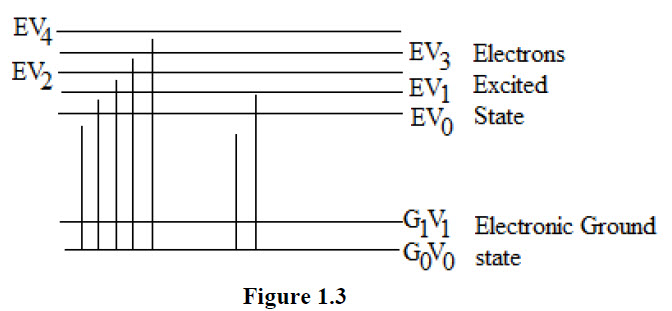
The types of electrons present in organic molecules may be classified as.
(a) α-Electrons: these electrons are involved in saturated bonds such as those between carbon & hydrogen in paraffin. As the amount of energy required exciting electrons in s bands is much more than that produced by UV light, compounds containing s bands do not absorb UV radiation. For this reason, paraffin’s compounds are frequently very useful as solvents.
(b) π-electrons: these electrons are involved in unsaturated hydrocarbons, the compounds with double or triple bonds and aromatic compounds.
(c) n-electrons: these are the electrons which are not involved in any of the bonding between atoms in molecules . Examples are organic compounds containing nitrogen, oxygen, or halogens. As n electrons can be excited by UV radiation, any compound that contains atoms like nitrogen, oxygen, sulphur, halogen compounds or unsaturated hydrocarbons may absorb UV radiation.
Following information may be sought regarding structure of organic compounds
1. Detection of conjugation: It helps to show the relationship between different groups, particles with respect to conjugation; the conjugation may be (a) between two or more carbon-carbon double or triple bonds, (b) between carbon-carbon and carbon oxygen double bonds or (c) between double bond and an aromatic ring. It can reveal the presence of an aromatic ring itself and the number and locations of Substituents attached to the carbons of the conjugated system.
2. Detection of geometrical isomers: In case of geometrically isomeric compounds, the Trans isomer exhibit λmax at slightly longer wavelength’s and have larger extinction coefficients than the cis isomers. For example, of the two stilbenes (C6H5CH=CH-C6H5), the Trans isomer show λmax= 294 nm while the cis isomer has λmax = 278 nm.
3. Detection of functional groups: It is possible to detect the presence of certain functional groups with the help of UV spectrum. Even the absence of any absorption above 200 nm is of some utility since it shows the absence of conjugation, carbonyl group and benzene rings in the compound. The earliest example, where considerable information could be deduced from a UV spectrum was that of mycomycin, an antibiotic having the formula C13H10O2. It absorbs 8 molar proportions of hydrogen in presence of a catalyst and hence 8 multiple bonds are present. It was shown that it is having a non-conjugated –COOH group and a terminal triple bond. Ring unsaturation index is 9, which is accounted by 8 multiple bonds and the –COOH group. Hence it is acyclic. Examination of the possible chain structures which can accommodate 8 multiple bonds makes it evident that mycomycin is very likely to be a straight chain compound because branches would give rise to very reactive structures.









