Q.1. (a)Define and exemplify chromophores. Discuss their interaction with UV- visible radiation and illustrate their use in structural analysis of organic compounds.
Ans.1. (a) CHROMOPHORE: The term chromophore was previously used to denote a functional group of some other structural feature of which gives a color to compound. For example- Nitro group is a chromophore because its presence in a compound gives yellow color to the compound. But these days the term chromophore is used in a much broader sense which may be defined as “any group which exhibit absorption of electromagnetic radiation in a visible or ultra-visible region “It may or may not impart any color to the compound. Some of the important chromophores are: ethylene, acetylene, carbonyls, acids, esters and nitrile groups etc. A carbonyl group is an important chromophore, although the absorption of light by an isolated group does not give rise to any colour in the ultra-violet spectroscopy.
[adsense:336x280:8701650588]
Types of chromophores:
Two types of chromophores are known.
1. Chromophores in which the groups have π electrons undergo π-π* transitions. For examples:-ethylenes, acetylenes etc.
2. Chromophores having both π- electrons and n (non-bonding) electrons undergo two types of transitions. i.e., π-π* and n-π*, for examples: - carbonyls, nitriles, azo compounds and nitro compounds etc.
Identification of chromophores:
There is no set rule for the identification of a chromophore. The change in position as well as the intensity of the absorption depends upon a large number of factors. Following points may be useful.
1. Spectrum having a band near 300 mµ may possess two or three conjugated units.
2. Absorption bands near 270-350 mµ with very low intensity ɛmax 10-100 are because of n-π* transitions of carbonyl group.
3.Simple conjugated chromophores likes dienesor,α β –unsaturated ketones have εmaxvalues, i.e., from 10,000 to 20,000.
4.The absorption with ɛmax value between1, 000-10,000 reveals the presence of an aromatic system. If aromatic nucleus is substituted with groups which can extends the chromophore, the absorption take place at still higher value of extinction coefficients.
AUXOCHROMES: It is a group which itself does not act as a chromophore but when attached to a chromophore, it shifts the adsorption towards longer wavelength along with an increase in the intensity of absorption. Some commonly known auxochromic groups are: -OH, -NH2, -OR, -NHR, and –NR2. For example:- When the auxochrome –NH2 group is attached to benzene ring. Its absorption change from λ max 225 (ɛmax 203) to λmax 280 (εmax1430). All auxochromes have one or more non-bonding pairs of electrons. If an auxochromes is attached to a chromophore, it helps is extending the conjugation by sharing of non-bonding pair of electrons as shown below.
CH2 = CH – NR2---------------->CH2-CH-NH2
The extended conjugation has been responsible for bathochromic effect of auxochromes.
INTERACTION WITH UV- RADIATION:
When chromophores and auxochromes interact with UV-radiation gives varies characteristics given below:-
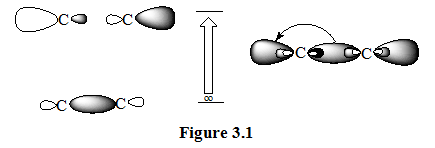
(1). ALKANES: For molecules, such as alkanes, that contain nothing but single bonds and lack atoms with unshared electrons pairs, the only electrons transitions possible are of the σ → σ* types. These transitions are of a high energy that they absorb ultraviolet energy at very short wavelength – shorter than the wavelengths that are experimentally accessible using typical spectrophotometers. Figure 1.1 illustrates this type of transition. The excitation of the σ- bonding electron to the σ*- anti bonding orbital is depicted at the right.
(2).ALCOHOLS, ETHERS, AMINES AND SULFUR COMPOUNDS: In saturated molecules that contains atoms bearing nonbonding pairs of electrons, transitions n-σ* of the type become important .They are also rather than high-energy transitions, but they do absorb radiation that lies within an experimentally accessible range. Alcohols and amines absorb in the range from 175 to 200 nm. While organic thiols and sulfides absorb between 200 and 220 nm. Most of the absorption is below the cutoff points for the common solvents, so they are not observed in solution spectra. Figure 1.2 illustrate an n-σ* transition for an amine. The excitation of the non-bonding electrons to the anti-bonding orbital is shown at the right.
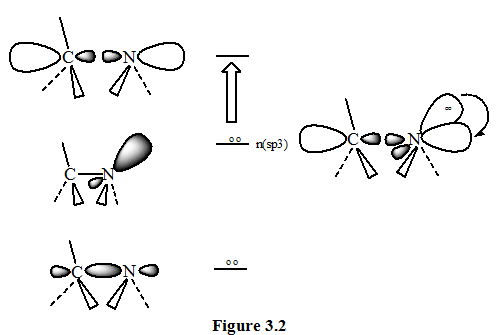
Ethanal can therefore absorb light of two different wavelengths:
• The pi bonding to pi anti-bonding absorption peaks at 180 nm;
• The non-bonding to pi anti-bonding absorption peaks at 290 nm.
Both of these absorptions are in the ultra-violet, but most spectrometers won't pick up the one at 180 nm because they work in the range from 200 - 800 nm.
(3). ALKENES AND ALKYNES: With unsaturated molecules, π-π* transitions become possible. These transitions are of rather high energy as well, but their positions are sensitive to the presence of substitution, as well be clear later , alkenes absorb around 175nm and alkynes absorb 170 nm Figure 1.3 shows this types of transitions.
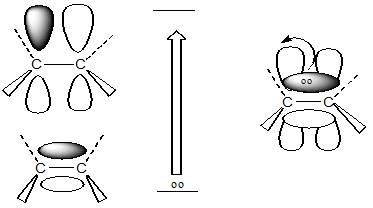
Figure 3.3
CARBONYL COMPOUND: Unsaturated molecules that contain atoms such as oxygen or nitrogen may also undergo n-π* transitions. These are perhaps the most interesting and most studied transitions particularly among carbonyl compounds. These transitions are also rather sensitive to substitution on the chromophoric structure. The typical carbonyl compounds undergoes an n-π* transitions around 280-290 nm. (ɛ = 15). Most n-π* transitions are forbidden and hence are of low intensity. Carbonyl compounds also have a π-π* transitions.

The non-bonding orbital has a higher energy than a pi bonding orbital. That means that the jump from an oxygen lone pair into a pi anti-bonding orbital needs less energy. That means it absorbs light of a lower frequency and therefore a higher wavelength









