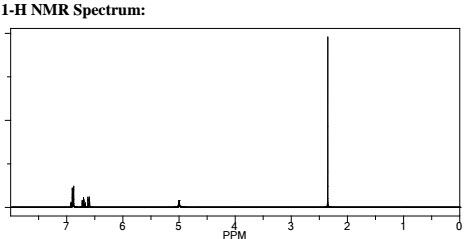
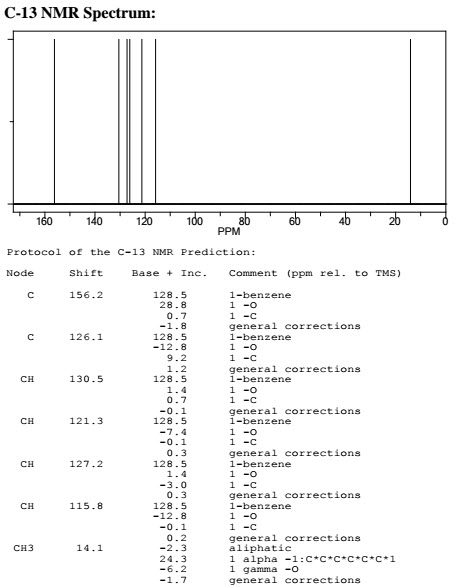
Q.1. (a) How 1HNMR spectra is different from 13C? Explain with suitable example.
Ans.1. (a)Difference between 13C-NMR and 1HNMR:
[adsense:336x280:8701650588]
|
S.NO. |
13C-NMR |
1HNMR |
|---|---|---|
|
1 |
Study of spin changes of carbon nuclei. |
Study of spin changes of proton nuclei. |
|
2 |
Chemical shift ranges between 0-240 ppm. |
Chemical shift ranges 0-14 ppm |
|
3 |
Very fast process |
Very low process |
|
4 |
Pulse-Fourier transform technique is used |
Continuous wave method is used. |
|
5 |
Valve of coupling constant ranges from 125-250 HZ |
Ranges from 0-15 HZ |
|
6 |
Information of total number of protons carbons is obtained. |
Information like total number, types of chemical environment of protons is obtained. |
|
7 |
By peak splitting, possible to interpret protons directly attached to carbon atom |
It possible to interpret protons attached to adjacent carbon atom |
|
8 |
Effect of substitute on substitute on adjacent carbon atom cannot vary the chemical shift. |
Substituent alter the chemical shift. |
|
9 |
It works on frequency sweep |
It works on either field sweep or frequency sweep. |
Examples:

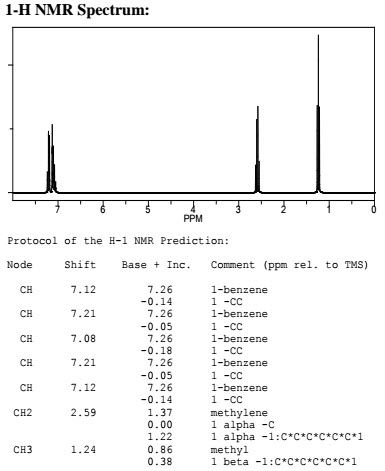

(ii)