Q.2. (a) Write an exhaustive note on thermogravimetry
Ans.2. (a) Thermo gravimetric analysis:
[adsense:336x280:8701650588]
In a thermo gravimetric analysis (TGA) consist of
1. A sensitive microbalance, called a Thermo balance
2. A furnace
3. A purge – gas system for providing an inert, or sometimes reactive, atmosphere ;
4. A computer system for instrument control, data acquisition, and data processing. A purge-gas switching system is a common option for application in which the purge gas must be changed during an experiment.
Thermo balance A number of different thermo balance design available commercial commercially are capable of providing quantitative information about sample ranging in mass from less than 1 mg to 100 g. the usual range of thermo balances. However, is form 1 to 100 mg. many of the balances can detect changes in mass as small as 0.1 μg. although the sample holder must be housed in the furnace, the rest of the balance must be thermally isolated from the furnace represented as fig. 1.4.
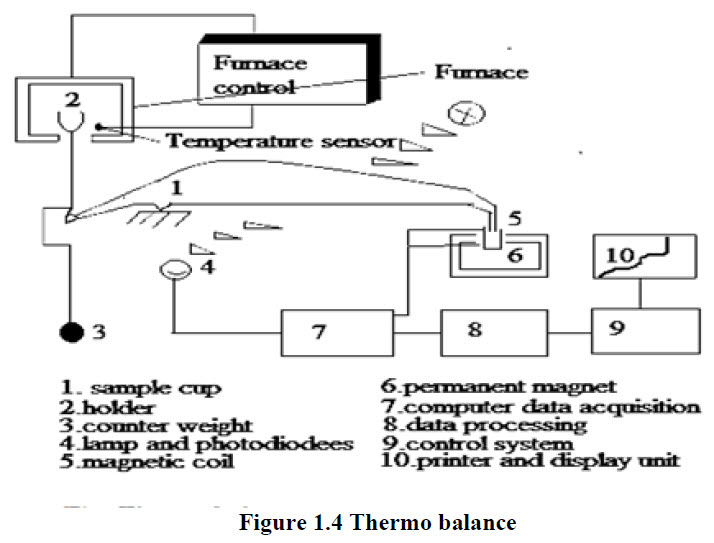
Sample holder
Samples are typically contained in sample pans made of platinum, aluminium, or alumina. Platinum is most often used because of its inertness and ease of cleaning. The volumes of sample pans range from 40 μL to more than 500 μL. Auto samplers are available as attachments for most TGA systems. With the majority of these units, all aspects are automated under software control. The sample pan tarring, loading, and weighing; the furnace heating and cooling; and the pan unloading are totally automatic.
Temperature Control and Data Processing
The temperature recorded in a thermo gram is ideally the actual temperature of the sample. This temperature can, in principle, be obtained by immersing a small thermocouple directly in the sample. Such a procedure is seldom followed, however, because of possible catalytic decomposition of samples, potential contamination of samples, and weighing errors resulting from the thermocouple leads. Because of these problems, recorded temperatures are generally measured with a small thermocouple located as close as possible to the sample container. The recorded temperatures then generally lag or lead the actual sample temperature.
Modern TGA systems use a computerized temperature control routine that automatically compares the voltage output of the thermocouple with a voltage-versus-temperature table stored in computer memory. The computer uses the difference between the temperature of the thermocouple and the temperature specified to adjust the voltage to the heater.
Combined Thermal Instruments
Several manufacturers offer systems that provide simultaneous measurement of heat flow and mass change or of energy change and mass change. Such instruments can not only track the loss of material or a vaporization phenomenon with temperature but also reveal transitions associated with these processes. These combination units can eliminate the effects of changes in sample size, homogeneity, and geometry. Many TGA systems produce the derivative of the thermogram as well as the thermogram itself. Such derivative plots are not true differential thermograms as produced in differential thermal analysis, but they provide similar qualitative information. They are often called single differential thermal analysis (SDTA) plots.
TGA/MS and TGA/FTIR
TGA is used to determine the loss in mass at particular temperatures, but TGA cannot identify the species responsible to obtain this type of information, the output of a thermogravimetric analyzer is often connected to a Fourier transform infrared (FTIR) or a mass spectrometer (MS). Several instrument companies offer devices to interface the TGA unit to a spectrometer. Some even claim true integration of the software and hardware of the TGA/MS or TGA/FTIR systems.
High-Resolution TGA
In high resolution TGA, the sample heating rate fluctuates so that the sample is heated more rapidly during periods of constant mass than during periods when mass changes occur. This allows higher resolution to be obtained during the interesting periods and reduces the time of inactivity.
High-Resolution TGA
In high resolution TGA, the sample heating rate fluctuates so that the sample is heated more rapidly during periods of constant mass than during periods when mass changes occur. This allows higher resolution to be obtained during the interesting periods and reduces the time of inactivity. Applications Thermo gravimetric analysis:
1. Because TGA monitors the mass of the analyte with temperature, the information provided is quantitative, but limited to decomposition and oxidation reactions and to such physical processes as vaporization, sublimation, and desorption. Among the most important applications of TGA are compositional analysis and decomposition profiles of multicomponent systems.
2. In Polymer studies, thermograms provide information about decomposition mechanisms for various polymeric preparations. In addition, the decomposition patterns are characteristic for each kind of polymer and can sometimes be used for identification purposes.
3. Polyethylene sample has been formulated with fine carbon black particles to inhibit degradation from exposure to sunlight. This analysis would be difficult by most other analytical methods.
4. Automatic thermogravimetric analysis: This is one of the most important applications of thermogravimetry in analytical chemistry.
5. Testing of purity of samples: As an analytical tool, the thermobalance can be used in determining the purity of various substances. This can be illustrated by considering the following example: Newkirk in his research paper established how an impure sample of calcium oxalate showed an unusual weight loss below 100◦c. When calculations were carried out, it was observed that the weight loss was not due to adsorbed water but due to some impurity.
6. Curie point determination: If a ferromagnetic material is kept on a thermobalance with the pole of a magnet above the sample, the TG balance will show the weight which will be less than the actual weight. Ferromagnetic materials loss their magnetism on heating at exactly reproducible temperatures on Curie points. A range of metals or alloys with Curie points between 150◦c and 1000◦c is available.
7. Other Applications: One of the first important applications of thermogravimetry was the determination of correct drying temperatures for precipitates used in gravimetric analysis. A second important application was the identification of the gases given off while a sample’s temperature increases. In addition, the composition of the residue can be determined. This information reveals the chemical decomposition process of heated materials and permits identification of the formulas of the residue. A third application of TG is the identification of the compound present in mixtures of materials. When such mixtures are heated using a thermogravimeter, a thermogram is produced for each component. These thermograms are superimposed on each other to provide a single composite thermogram for the sample. Interpretation of the complete thermogram requires that the individual thermograms be separated and identified. Not only the components of the mixture be identified, but a quantitative determination of each is possible from the thermogram.









