About Author:
Patelia Emanual Michael
Department of pharmacology, University of Bedfordshire,
United Kingdom, LU1 3JU.
ricky.emanual@gmail.com
Abstract:
Aspirin continues to be evaluated in vitro and in pre-clinical models to help elucidate mechanisms involved in carcinogenesis and the response of tumours to anti-neoplastic agents. Recent randomised evidence from trials primarily designed to prevent cardiovascular disease show a reduction in cancer incidence with long-term follow-up and epidemiological evidence from colorectal and breast cancer studies evaluating the effects of aspirin use after diagnosis suggests that aspirin may have a role in the adjuvant setting. The clinical management of patients is also continually evolving, with new combinations of agents or strategies being assessed; aspirin should not be overlooked in this process because it is neither new nor expensive.
REFERENCE ID: PHARMATUTOR-ART-1800
INTRODUCTION:
Clinical guidelines on behalf of prophylactic aspirin use presently merely regarded as the cardiovascular benefits of aspirin, weighed against the potential harm from aspirin-induced bleeding. Daily aspirin use has been convincingly shown to reduce the risk of colorectal cancer and recurrence of adenomatous polyps, but in average-risk populations, these benefits alone do not outweigh harms from aspirin-induced bleeding. Recently published secondary analyses of cardiovascular trials provide the first randomized evidence that daily aspirin use may also reduce the incidence of all cancers combined, even at low doses (75–100 mg daily). This Review considers the general mechanism of action that defines aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) as a class, the specific advantages of aspirin over other NSAIDs for prophylactic use, the recent evidence concerning the main health outcomes affected by aspirin use, and the hypothesis that inhibition of platelet activation may mediate both the cardioprotective and cancer-preventive effects of low-dose aspirin. It also considers how even a 10% reduction in overall cancer incidence beginning during the first 10 years of treatment could tip the balance of benefits and risks favourably in average-risk populations.
Current drug development work recognises that the growth of tumours involves cross-talk between different signalling pathways, and that resistance develops to agents that have a single target. Aspirin affects multiple intracellular pathways and influences physiological processes such as apoptosis and angiogenesis that are important in the growth and development of malignancies (Figure 1). Publicly funded researchers have a responsibility to ensure that drugs for which there is no longer a financial incentive for pharmaceutical companies to develop further are assessed in light of current knowledge and evolving clinical practice. Aspirin pre-dates current anti-cancer strategies such as the use of adjuvant chemotherapy after a potentially curative operation. Although significant tumour shrinkage is not seen when aspirin is administered for other clinical indications such as cardiovascular disease, epidemiological evidence and pre-clinical data suggest that aspirin is worthy of further investigation particularly in the adjuvant setting, after potentially curative surgery and chemotherapy if appropriate, when disease burden is expected to be minimal. Regular aspirin use is not currently recommended as a primary prevention strategy against cancer for those at average risk because of the risk of toxicity, particularly serious gastrointestinal bleeding. It is estimated that regular aspirin use increases the risk of a significant bleed from 1% over 10 years to 2–3% (Cuzick et al, 2009) and this outweighs the potential cancer benefits particularly if effective screening is available. For aspirin administered adjuvantly, the benefit:risk ratio will be different, as higher morbidity and mortality from recurrent cancer may outweigh the toxicity associated with regular aspirin use. There is also potential for wider health benefits; colorectal cancer shares similar risk factors, such as smoking and the metabolic syndrome, with coronary artery disease; thus, aspirin could potentially be beneficial from both an oncological and cardiological perspective (Chan et al, 2007). In any future trials the challenge will be to identify and exclude those individuals most at risk of toxicity, for example, those with a previous history of gastric ulceration (Patrono et al, 2001) and include those most likely to benefit. Commencing aspirin while conventional adjuvant cytotoxic chemotherapy is being administered could increase toxicity, particularly the risk of bleeding if thrombocytopaenia was present. Waiting until chemotherapy had finished would allow the use of a ‘run-in’ period, as used in adenoma prevention trials, in which a dose of 300 mg daily appeared to be well tolerated and participants were assessed as to whether they would be able to tolerate aspirin before they were randomised (Baron et al, 2003; Sandler et al, 2003). This increased compliance and reduced the risk of serious adverse events particularly gastrointestinal haemorrhage. With the exception of the recent data from the Thrombosis Prevention Trial and the Swedish Aspirin Low Dose Trial presented by Rothwell et al (2010), the epidemiological data and the randomised trials assessing primary prevention support the premise that the anti-cancer effects of aspirin are most likely to be seen when higher doses are administered, there is long-term use (many years), longer follow-up (410 years in some instances) and daily usage rather than alternate day scheduling. At higher doses, aspirin is a more potent inhibitor of Cox-2 providing a potential mechanistic explanation for these findings. The observation that the benefit of aspirin after colorectal diagnosis was greatest in those whose tumours overexpressed Cox-2, and that those who had taken aspirin before diagnosis did not appear to benefit from taking aspirin adjuvantly (Rothwell et al, 2010) gives an indication as to who may benefit from aspirin after a cancer diagnosis and emphasises the need for pathological assessment of tumour samples to be built into any randomised trials. The current limited testing of aspirin (clinicaltrials.gov) as a therapeutic agent either in the adjuvant setting (ASCOLT and Big A trial) or in combination with other anti-cancer agents is in marked contrast to the number of studies that were initiated using selective Cox-2 inhibitors before 2004. There were numerous phase II studies and at least 12 randomised phase III trials that were ongoing in 2004, before the concerns about cardiovascular toxicity were raised, with 49000 planned participants including those with breast, colorectal, oesophageal, prostate and lung malignancies. A number of these studies involved rofecoxib and had to be discontinued when the product was withdrawn. Others were stopped early although the investigational agent (usually celecoxib) was not withdrawn.
Aspirin continues to be evaluated in vitro and in pre-clinical models to help elucidate mechanisms involved in carcinogenesis and the response of tumours to anti-neoplastic agents. Recent randomised evidence from trials primarily designed to prevent cardiovascular disease show a reduction in cancer incidence with long-term follow-up and epidemiological evidence from colorectal and breast cancer studies evaluating the effects of aspirin use after diagnosis suggests that aspirin may have a role in the adjuvant setting. The clinical management of patients is also continually evolving, with new combinations of agents or strategies being assessed; aspirin should not be overlooked in this process because it is neither new nor expensive.
REVIEW EVIDENCE ON CANCER PREVENTION (REVIEW OF LITURATURE)
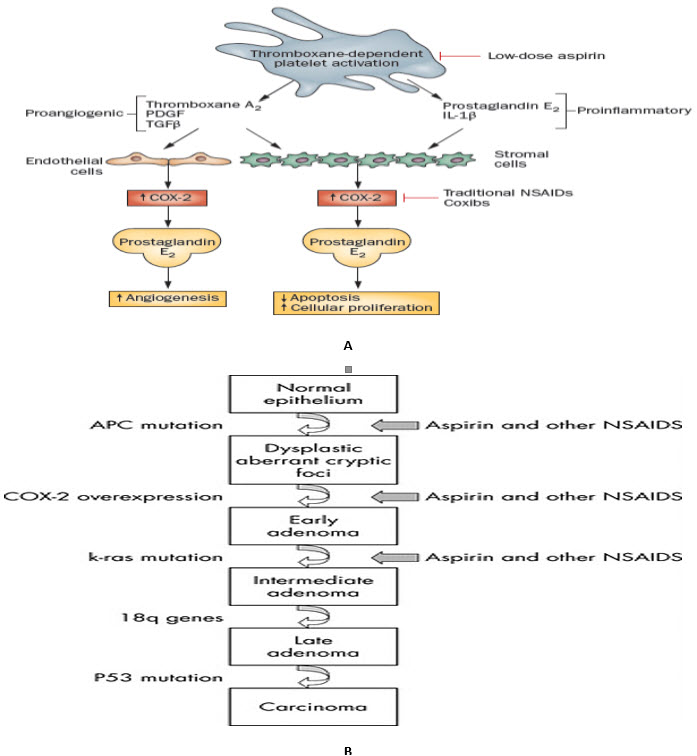
FIGURE 1: Multistep progression of colon cancer and sites of NSAID action.(B) (S. Sanghaet al., 2005) and Hypothesized mechanism by which the inhibition of COX?1 in platelets by low-dose aspirin may suppress the induction of COX?2 in adjacent nucleated cells of the intestinal mucosa in early stage neoplasia (A) (Thun M. J. et al., 2012).
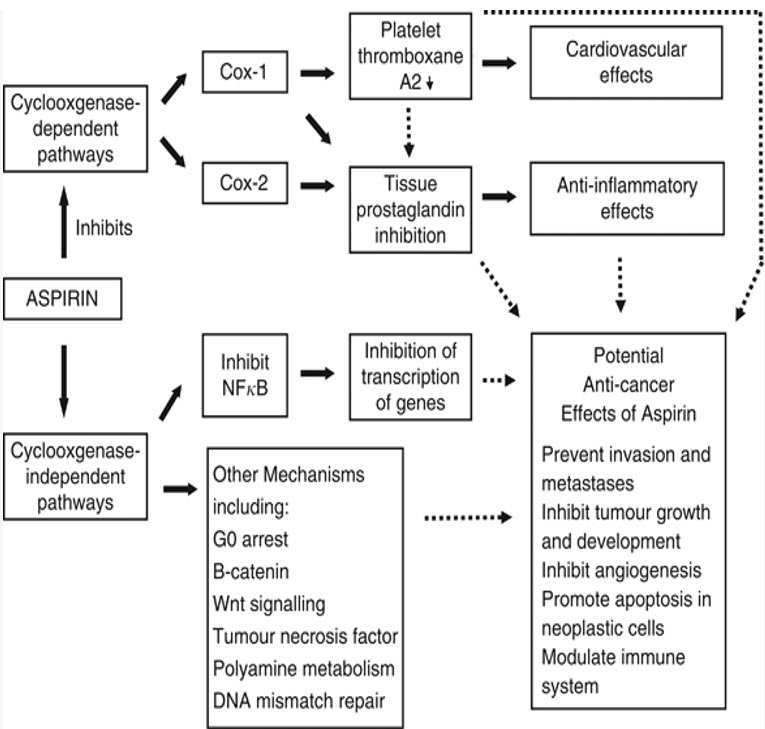
FIGURE 2: Aspirin mechanisms of action and pathophysiological effects. Black block arrows indicate known mechanisms. Dotted black arrows indicate potential mechanisms that could contribute to anti-cancer effects. Cox=cyclooxygenase; NFκB=nuclear factor-κB (Langley et al., 2011).
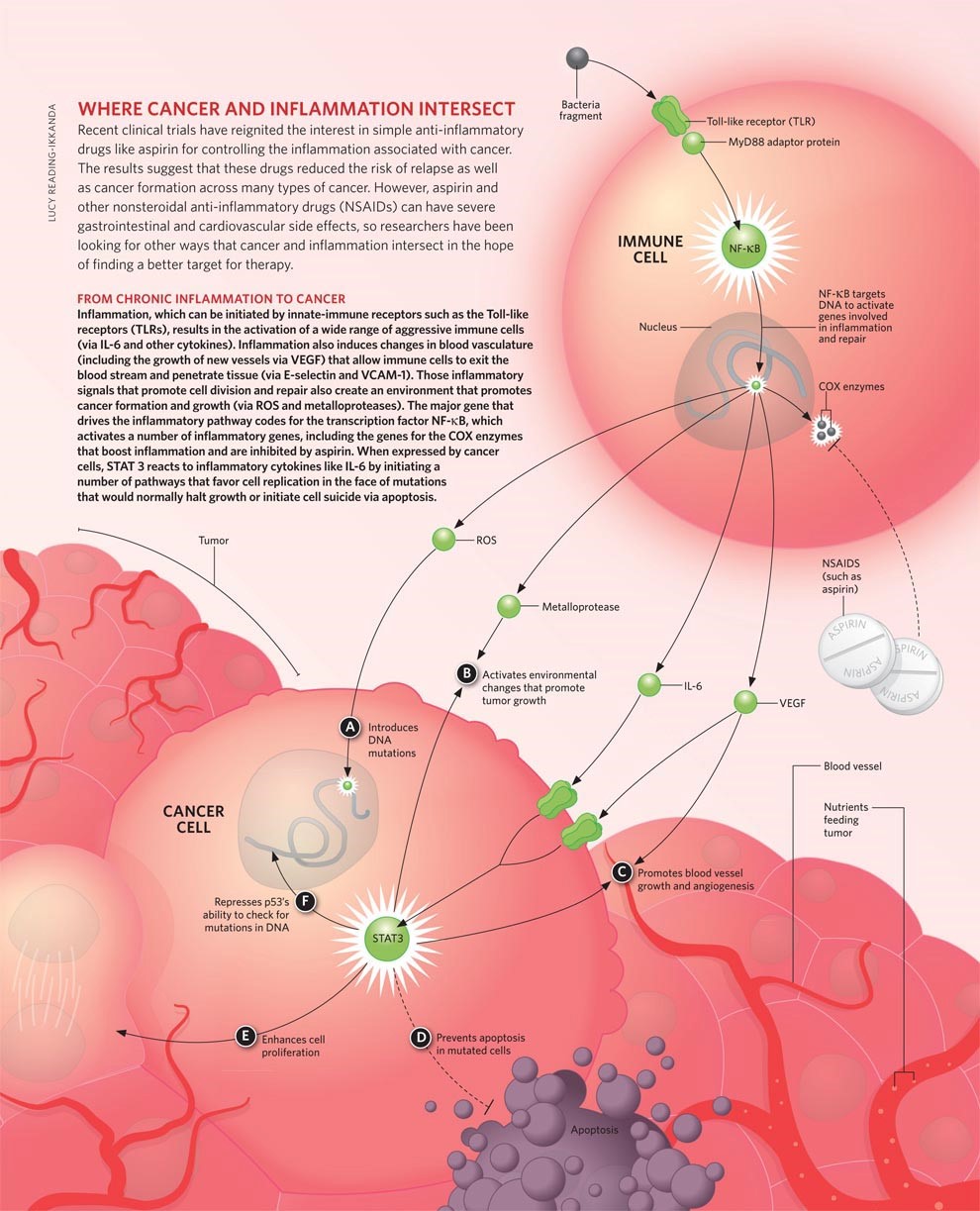
FIGURE 3: A DETAILED DEMONSTRATION AND MECHANISM OF ASPIRIN IN CANCER PREVENTION
* ASPIRIN: A ROLE MODEL IN CANCER PREVENTION CARE(REVIEW OF LITURATURE)
New evidence has shown that aspirin has a potential role in reducing the risk of cancer death—which could mean that clinical guidelines for the use of aspirin in preventive care might someday include cancer prevention, according to the results of a review published early online in Nature Reviews Clinical Oncology.[ Thun M.J. et al., 2012]
The current clinical guidelines for the use of aspirin in disease prevention consider only its cardiovascular benefits, weighed against the potential harm from aspirin-induced bleeding. In previous studies, daily aspirin use has been shown to reduce the risk of colorectal cancer and the recurrence of polyps; however, these benefits have not outweighed the potential harm from aspirin-induced bleeding.
Now, recently published secondary analyses of cardiovascular trials indicate that aspirin may have a viable role in cancer prevention. The data provides the first randomized evidence that daily aspirin use may also reduce the incidence of all cancers combined, even at low doses (75–100 mg daily) and also combination with other drugs like ambroxol HCl and doxophylline (Patelia et al., 2013). In fact, the evidence suggests that a daily dose of aspirin (75mg or more) might lower overall cancer incidence and overall cancer mortality.
The review included six primary prevention trials in which patients were randomized to daily low-dose aspirin treatment. The results indicated an approximately 20 percent reduction in overall cancer incidence between three and five years after initiation of aspirin use and a 30 percent reduction during follow-up more than five years later. Follow up that occurred more than five years after initiation of aspirin use indicated that cancer mortality was also reduced. The observed benefit did not increase with aspirin doses above 75-100mg.
Notably, the data from this review excluded results from the Women’s Health Study (WHS), a 10-year trial in which women took 100mg of aspirin ever other day and which reported no reduction in cancer incidence or mortality.
Researchers continue to study the risks and benefits of daily aspirin use, but it appears that evidence is mounting that aspirin may have a valid preventive role in cancer. The researchers noted that even a 10 percent reduction in overall cancer incidence during the first 10 years of aspirin treatment could affect the risk-benefit balance—meaning the benefits of aspirin could outweigh the risks in average-risk populations. Stay tuned—as evidence accumulates, the clinical guidelines for the preventive use of aspirin could change to include cancer prevention.
* UPDATE ON ASPIRIN AND CANCER PREVENTION(REVIEW OF LITURATURE)
We were very interested in the role of aspirin in preventing cancer. We did a consensus statement [Cuzick J. et al 2009] about 2 years ago in which we said the data looked very promising, but what was needed was longer follow-up of the ongoing trials. Much of that has now taken place, and some new results have come out, so the results look even more promising.
What is particularly clear is that the effect of aspirin on prevention is a long-term effect and that not very much happens in the 5 years after you start taking aspirin. The preventive effects are really quite long-term; that is very important in terms of how it should be used, if you are going to need to start 5 years before there is going to be any benefit.
There have been 2 recent papers. One was on patients with Lynch syndrome, which puts them at high risk for colorectal and a few other cancers. That was a trial of both starch and aspirin, and the initial results [Burn J. et al., 2008] when they were published a few years ago were actually negative for both of these. There was no effect at all, and now with additional follow-up, [Burn J. et al., 2011] we have seen what was seen in many other studies or was emerging in many other studies: that the effects take a long time to kick in, so now there is a substantial reduction in cancer, particularly colorectal cancer, but some additional cancers as well.
This was a high-dose trial, so again, it doesn't actually address directly the question of whether low-dose aspirin is appropriate. The dose that was tested was 600 mg/day (2 standard aspirin tablets per day), so there were many questions about how best to use aspirin both in the general population and in patients at high risk. The dose is one question.
There was another recent paper from Professor Rothwell and colleagues [Rothwell P.M. et al. 2011] looking at a whole range of cancers in the randomized trials, and again, the same feature showed up -- that the effect is quite long-term. Not very much happened in the first 5 years of treatment, but after that, preventive effects emerge for a number of cancers. The most striking were, again, colorectal cancer, esophageal cancer, and to some extent stomach cancer. So it looks like all of the gastrointestinal cancers might be quite substantially affected, with maybe as much as a one-third reduction with long-term aspirin use.
There also was emerging evidence for a few other cancers, notably breast cancer, ovarian cancer, lung cancer, and prostate cancer. The evidence is less clear and less striking, but overall the deaths from cancer in these studies were reduced by 20%, so there is something going on above and beyond just what was seen in the colon cancer study.
There are many possible mechanisms. It could be as simple as the fact that aspirin reduces inflammation, and inflammation is associated with more rapid cell turnover. The more times a cell divides, the greater the chance for an error that could lead to a mutation leading to cancer. It could be as simple as that. Many explanations are more complicated as well. There is plenty of room for research, both at the mechanistic level in terms of trying to understand what is going on, and more applied research, in trying to figure out what is the best way that we can live with low-dose aspirin for cancer prevention. The data from the Rothwell paper suggest that the low dose may be as effective as the higher dose, but we probably need more evidence to be quite certain about that.
The other major issue is at what age to start and stop taking aspirin, and that is very much a balance between the benefits in terms of cancer and heart disease and the risks in terms of gastrointestinal bleeding. There is emerging evidence that bleeding effects are only serious in individuals over the age of 70 years and that typically in younger people, they aren't so serious and they tend to disappear after stopping treatment, without any serious problems. That needs to be looked at more, but it does suggest that one should maybe be thinking about starting aspirin around age 50-55 years, and maybe taking it for 5-10 years and then trying to finish taking it before you get to age 65-70 years, when the side effects are potentially more serious. That is very much an area for research. We don't really have clear answers, but those are suggestions.
The other major area for research is trying to identify subpopulations at increased risk for gastric bleeding and trying to find ways to either avoid aspirin in that group or address the issues. One of the things that is striking and quite simple is that there is substantial evidence that the bleeding and the ulcers caused by aspirin are a particular problem in people who have Helicobacter pylori infection, so maybe a simple thing like testing and eradicating that organism before you start aspirin therapy might go a long way toward reducing those side effects.
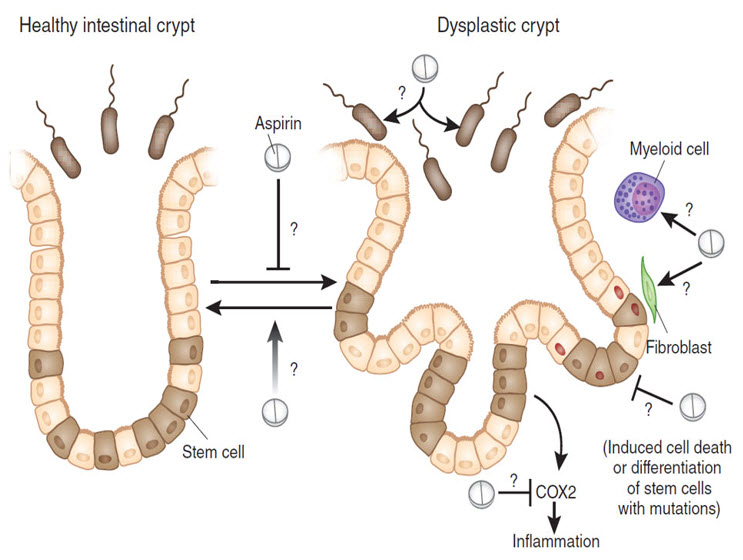
FIGURE 4: Two years of aspirin treatment can reduce the risk of developing colorectal cancer in individuals with Lynch syndrome several years after treatment. Although how aspirin acts to prevent cancer progression is still unknown, one hypothesis points at the targeting of intestinal stem cells, thus acting very early on in the path toward malignancy. But aspirin may also have a chemoprevention effect by acting on other host cells or the gut microbiota and also with other chemicals like nitazoxanide (Patelia et al., 2012). Further investigation will be needed to fully understand how aspirin can prevent colorectal cancer in predisposed individuals and the clinical value of this treatment in other populations. Red nuclei indicate the presence of a mutation
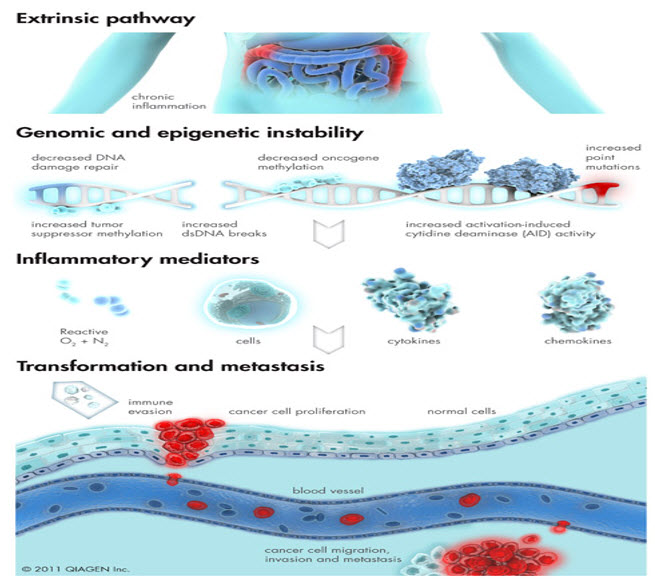
FIGURE 5: Inflammation of external origin is responsible for increased cancer risk via induction of genetic and epigenetic aberrations in affected cells.
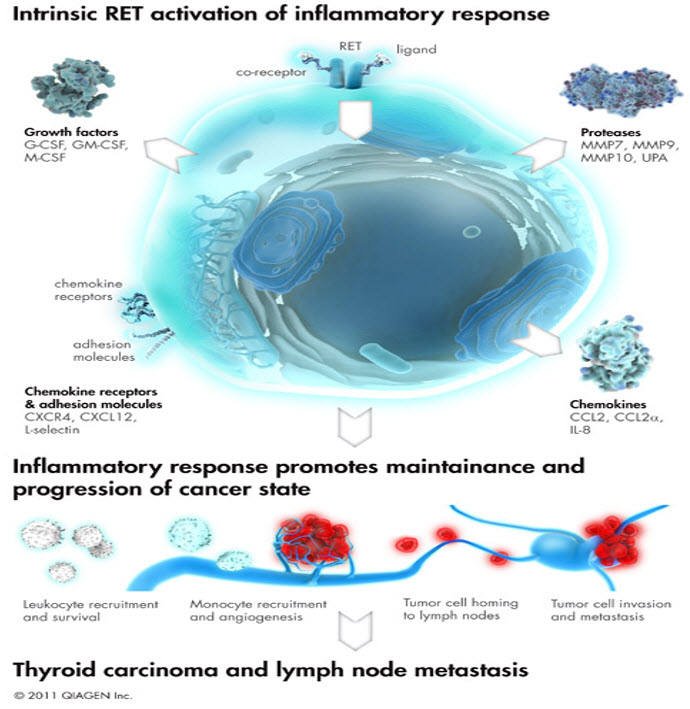
FIGURE 6: RET oncogenic signaling pathway activation is a well characterized example for the intrinsic origin of cancer inflammation.Aberrant activation of certain oncogene signaling pathways can elicit inflammatory responses that are needed to maintain cancer cell survival and progression. This figure depicts the mechanism of protein tyrosine kinase oncogene, RET, induced inflammation in thyroid cancer.
REFERENCES:
- Baron J.A., Cole B.F., Sandler R.S., Haile R.W., Ahnen D., Bresalier R., McKeown- Chan A.O., Jim M.H., Lam K.F., Morris J.S., Siu D.C., Tong T., Ng F.H., Wong S.Y., Hui W.M., Chan C.K., Lai K.C., Cheung T.K., Chan P., Wong G., Yuen M.F., Lau Y.K., Lee S., Szeto M.L., Wong B.C., Lam S.K. (2007) Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 298 1412–1419
- Burn J., Bishop D.T. and Mecklin J.P. (2008) CAPP2 Investigators. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 359 2567-2578.
- Burn J., Gerdes A.M. and Macrae F. (2011) Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet. 378 2081-2087.
- Chan A.T., Giovannucci E.L., Meyerhardt J.A., Schernhammer E.S., Curhan G.C., Fuchs C.S., (2005) Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294 914–923
- Chan A.T., Giovannucci E.L., Meyerhardt JA, Schernhammer E.S., Wu K. and Fuchs C.S. (2008) Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 134 21–28
- Chan A.T., Ogino S. and Fuchs C.S. (2009) Aspirin use and survival after diagnosis of colorectal cancer. JAMA 302 649–658
- Cuzick J., Otto F., Baron J.A., Brown P.H., Burn J., Greenwald P., Jankowski J., La Vecchia C., Meyskens F., Senn H.J. and Thun M. (2009) Aspirin and nonsteroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10 501–507
- Cuzick J., Otto F. and Baron J.A. (2009) Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10 501-507.
- Jacobs E.J. and Patrono C. (2012) The role of aspirin in cancer prevention. Nature Reviews Clinical Oncology 1 199.
- Langley R.E., Burdett S., Tierney J.F., Cafferty F., Parmar M.K.B. and Venning G. (2011) Aspirin and cancer: has aspirin been overlooked as an adjuvant therapy? Britis J Cancer 105 1108
- Patelia E. M., Falgun A Mehta and Pradip N Bhoya (2013) Simultaneous Estimation of Ambroxol Hydrochloride and Doxofylline in Pharmaceutical Formulation by HPTLC-Desitometric Method J Chrom Sep Tech 4 1
- Patelia E. M., Keyur B.A. and Falgun A.Mehta (2012) Development of a Validated Stability- Indicating HPTLC Method for Nitazoxanide in Pharmaceutical Formulation J Chrom Sep Tech 3 1
- Patrono C., Patrignani P., Garcia Rodriguez L.A. (2001) Cyclooxygenaseselective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest 108 7–13
- Rothwell P.M., Fowkes F.G., Belch J.F., (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 377 31-41.
- Rothwell P.M., Wilson M., Elwin C-E, Norrving B., Algra A., Warlow C.P., Meade T.W. (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376 1741–1750
- S Sangha, M Yao, M M Wolfe (2005) Non-steroidal anti-inflammatory drugs and colorectal cancer prevention Post grad Med J 81 223-227
- Sandler R.S., Halabi S., Baron J.A., Budinger S., Paskett E., Keresztes R., Petrelli N., Pipas J.M., Karp D.D., Loprinzi C.L., Steinbach G., Schilsky R. (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348 883–890.
- Thun, M. J. (2012) Role of aspirin in cancer prevention Nat. Rev. Clin. Oncol. 9 259–267
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

