About Authors:
Kataria Sahil,Middha Akanksha, Sandhu Premjeet
Seth G. L. Bihani S.D. College of Technical Education,
Institute of Pharmaceutical Sciences and Drug Research,
Sri Ganganagar, Rajasthan, INDIA
ABSTRACT
Method validation is the process used to confirm that the analytical procedure employed for a specific test is suitable for its intended use. Results from method validation can be used to judge the quality, reliability, and consistency of analytical results; it is an integral part of any good analytical practice. When extended to an analytical procedure, depending upon the application, it means that a method works reproducibly, when carried out by same or different persons, in same or different laboratories, using different reagents, different equipment etc. In this review article we discussed about the strategy and importance of validation of analytical methods.
Reference ID: PHARMATUTOR-ART-1172
1. Introduction
Process validation is a requirement of current Good Manufacturing Practices (GMPs) for finished pharmaceuticals and of the GMP regulations for medical devices and therefore applies to the manufacture of both drug products and medical devices.9
* According to USP General chapter <1225> “Validation is the process of providing documented evidence that the method does what it is intended to do” In other words the process of method validation ensures that the proposed analytical methodology is accurate, specific, reproducible and rugged for its intended use 2
* According to the FDA Guidelines on General Principles of Process Validation, process validation is defined, ‘‘ as establishing documented evidence, which provides a high degree of assurance, that a specific process will consistently produce a product meeting its predetermined specifications and quality characteristics.’’ The process for making a drug product consists of a series (flow diagram in logically defined steps) of unit operations (modules) that result in the manufacture of the finished pharmaceutical.9
* The elements “Quality control system” and “Validation and process Validation” of Schedule M to the drugs and cosmetic rules provide as under
“16.12 All instruments shall be calibrated and testing procedures validated before these are adopted for routine testing. Periodical calibration of instruments and validation of procedures should be carried out”
“26.1 Validation studies should be an essential part of GMP and shall be conducted as per the predefined protocols. These should include validation of Processing, testing and cleaning procedures”
* In WHO GMP under the element “Qualification and Validation”. Validation of analytical test methods, automated systems and cleaning procedure has been emphasized. It reads as under
“It is of critical importance that particular attention is paid to validation of analytical test methods, automated systems and cleaning procedures” 12
In International Pharmacopoeia the guidelines are directed primarily to the examination of chemical and physiochemical attributes, but many of the general principles are also applicable to microbiological and biological procedures16
According to ICH Guidelines Validation of an Analytical procedure is to demonstrate that it is suitable for its intended purpose 8
Guideline History
ICH Q2A :- Text on Validation of Analytical Procedures
ICH Q2B : - Guideline on Validation of Analytical Procedures: Methodology [ICH Q2(R1)]
ICH Q2R1 :- Q2A+Q2B 15
2. WHY VALIDATE ANALYTICAL PROCEDURES
There are many reasons for the need to validate analytical procedures. Among them are regulatory requirements, good science, and quality control requirements. The Code of Federal Regulations (CFR) 311.165c explicitly states that “the accuracy, sensitivity, specificity, and reproducibility of test methods employed by the firm shall be established and documented.”Of course, as scientists, we would want to apply good science to demonstrate that the analytical method used had demonstrated accuracy, sensitivity, specifi city, and reproducibility. Finally management of the quality control unit would definitely want to ensure that the analytical methods that the department uses to release its products are properly validated for its intended use so the product will be safe for human use.1
Analytical methods need to be validated, verified, or revalidated in the following instances:
- Before initial use in routine testing
- When transferred to another laboratory
- Whenever the conditions or method parameters for which the method has been validated change (for example, an instrument with different characteristics or samples with a different matrix) and the change is outside the original scope of the method.7
Furthermore revalidation may be necessary in the following circumstances:
- Changes in the synthesis of the drug substance;
- Changes in the composition of the finished product;
- Changes in the analytical procedure15
3. CYCLE OF ANALYTICAL METHODS
The analytical method validation activity is not a one - time study. This is illustrated and summarized in the life cycle of an analytical procedure in Figure
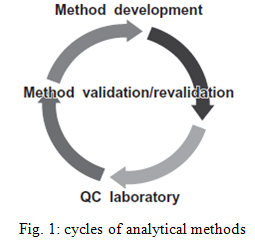
An analytical method will be developed and validated for use to analyze samples during the early development of an active pharmaceutical ingredient (API) or drug product. As drug development progresses from phase 1 to commercialization, the analytical method will follow a similar progression. The final method will be validated for its intended use for the market image drug product and transferred to the quality control laboratory for the launch of the drug product. However, if there are any changes in the manufacturing process that have the potential to change the analytical profile of the drug substance and drug product, this validated method may need to be revalidated to ensure that it is still suitable to analyze the API or drug product for its intended purpose. 6
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4. Validation Protocol
The text of 21 CFR Part 211, ICH guidelines and general chapter <1225> all provide terms and definitions, there is no specific discussion on Validation protocol and methodology. In ICH guidelines (Q2B) on method validation methodology, the applicant has been made responsible for appropriate validation protocol and procedure suitable for the product. Therefore prior to initiating a validation study, a well planned validation protocol is required. The validation protocol should include a detailed test procedure, basic experimental design, elements for validation, predefined acceptance criteria, reference or related methods and management approval.
A test procedure is description of the “analytical method” to be used as a guide in validating the method and serve as basis for preparation of validation protocol. It should include
- A listing of reagents, solvents and other supplies
- Instructions for preparation of standards, samples and solutions
- A listing of equipments to be used or equivalents
- Instrumental parameters and chromatographic conditions
- System suitability requirements
- Standard and sample analysis sequence
- Calculation sequence to include result formatting 2
5. Types of Analytical Procedures to be Validated
There are 3 types of analytical procedures:
· Regulatory analytical procedures
· Alternative analytical procedures
· Stability indicating assay
Regulatory analytical procedures are those procedures which are official in compendia of standards recognized by legislation of country for example, he drug and cosmetic rues recognize official procedures given in I.P. and other pharmacopoeia such as B.P.& U.S.P
Alternative analytical procedures are alternative procedures for regulatory analytical procedures. Generally pharmacopoeia state that alternative method can be used provided their performance is equivalent or more then pharmacopoeial analytical procedure.
Stability indicating assay is a validated quantitative method that can detect changes with time in particular properties of drug substance and drug product. It accurately measure the active ingredient without interference from degradation products, process impurities excipients or other potensial impurities 11
Validation of analytical procedures is directed to the four most common types of analytical procedures:
- Identification tests;
- Quantitative tests for impurities' content;
- Limit tests for the control of impurities;
- Quantitative tests of the active moiety in samples of drug substance or drug product or other selected component(s) in the drug product.
A brief description of the types of tests considered in this document is provided below.
- Identification tests are intended to ensure the identity of an analyte in a sample. This is normally achieved by comparison of a property of the sample (e.g., spectrum, chromatographic behaviour, chemical reactivity, etc) to that of a reference standard;
- Testing for impurities can be either a quantitative test or a limit test for the impurity in a sample. Either test is intended to accurately reflect the purity characteristics of the sample. Different validation characteristics are required for a quantitative test than for a limit test;
- Assay procedures are intended to measure the analyte present in a given sample. the assay represents a quantitative measurement of the major component(s) in the drug substance. For the drug product, similar validation characteristics also apply when assaying for the active or other selected component(s). The same validation characteristics may also apply to assays associated with other analytical procedures (e.g., dissolution).
Typical validation characteristics which should be considered are listed below:
1. Accuracy
2. Precision
2.1 Repeatability
2.2 Intermediate Precision
3. Specificity
4. Detection Limit
5. Quantitation Limit
6. Linearity
7. Range
The degree of revalidation required depends on the nature of the changes. Certain other changes may require validation as well.
TABLE 1 – Different Characteristics
|
Type of analytical procedure |
Identification |
Testing for impurities |
Assay - dissolution (measurement only) - content/potency |
|
characteristics |
|
quantitat. limit |
|
|
Accuracy |
- |
+ - |
+ |
|
Precision Repeatability Intermediate.Precision |
- - |
+ - + (1) - |
+ + (1) |
|
Specificity (2) |
+ |
+ + |
+ |
|
Detection Limit |
- |
- (3) + |
- |
|
Quantitation Limit |
- |
+ - |
- |
|
Linearity |
- |
+ - |
+ |
|
Range |
- |
+ - |
+ |
- signifies that this characteristic is not normally evaluated
+ signifies that this characteristic is normally evaluated
(1) in cases where reproducibility has been performed, intermediate precision is not needed
(2) lack of specificity of one analytical procedure could be compensated by other supporting analytical procedure(s)
(3) may be needed in some cases
6. DEFINITIONS
· ANALYTICAL PROCEDURE
The analytical procedure refers to the way of performing the analysis. It should describe in detail the steps necessary to perform each analytical test. This may include but is not limited to: the sample, the reference standard and the reagents preparations, use of the apparatus, generation of the calibration curve, use of the formulae for the calculation, etc.
· SPECIFICITY
Specificity is the ability to assess unequivocally the analyte in the presence of components which may be expected to be present. Typically these might include impurities, degradants, matrix, etc.
Lack of specificity of an individual analytical procedure may be compensated by other supporting analytical procedure(s).
This definition has the following implications:
Identification: to ensure the identity of an analyte.
Purity Tests: to ensure that all the analytical procedures performed allow an accurate statement of the content of impurities of an analyte, i.e. related substances test, heavy metals, residual solvents content, etc.
Assay (content or potency): to provide an exact result which allows an accurate statement on the content or potency of the analyte in a sample.
· ACCURACY
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. This is sometimes termed trueness.
· PRECISION
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility.
Precision should be investigated using homogeneous, authentic samples. However, if it is not possible to obtain a homogeneous sample it may be investigated using artificially prepared samples or a sample solution.
The precision of an analytical procedure is usually expressed as the variance, standard deviation or coefficient of variation of a series of measurements.
· Repeatability
Repeatability expresses the precision under the same operating conditions over a short interval of time. Repeatability is also termed intra-assay precision.
· Intermediate precision
Intermediate precision expresses within-laboratories variations: different days, different analysts, different equipment, etc.
· Reproducibility
Reproducibility expresses the precision between laboratories (collaborative studies, usually applied to standardization of methodology).
· DETECTION LIMIT
The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantitated as an exact value.
· QUANTITATION LIMIT
The quantitation limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy. The quantitation limit is a parameter of quantitative assays for low levels of compounds in sample matrices, and is used particularly for the determination of impurities and/or degradation products.
· LINEARITY
The linearity of an analytical procedure is its ability (within a given range) to obtain test results which are directly proportional to the concentration (amount) of analyte in the sample.
· RANGE
The range of an analytical procedure is the interval between the upper and lower concentration (amounts) of analyte in the sample (including these concentrations) for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and linearity.
· ROBUSTNESS
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
7. VALIDATION CHARACTRISTICS
7.1.SPECIFICITY
An investigation of specificity should be conducted during the validation of identification tests, the determination of impurities and the assay. The procedures used to demonstrate specificity will depend on the intended objective of the analytical procedure.
It is not always possible to demonstrate that an analytical procedure is specific for a particular analyte (complete discrimination). In this case a combination of two or more analytical procedures is recommended to achieve the necessary level of discrimination.
7.1.1.Identification
Suitable identification tests should be able to discriminate between compounds of closely related structures which are likely to be present. The discrimination of a procedure may be confirmed by obtaining positive results (perhaps by comparison with a known reference material) from samples containing the analyte, coupled with negative results from samples which do not contain the analyte. In addition, the identification test may be applied to materials structurally similar to or closely related to the analyte to confirm that a positive response is not obtained. The choice of such potentially interfering materials should be based on sound scientific judgement with a consideration of the interferences that could occur.
7.1.2.Assay and Impurity Test(s)
For chromatographic procedures, representative chromatograms should be used to demonstrate specificity and individual components should be appropriately labelled. Similar considerations should be given to other separation techniques.
Critical separations in chromatography should be investigated at an appropriate level. For critical separations, specificity can be demonstrated by the resolution of the two components which elute closest to each other.
In cases where a non-specific assay is used, other supporting analytical procedures should be used to demonstrate overall specificity. For example, where a titration is adopted to assay the drug substance for release, the combination of the assay and a suitable test for impurities can be used.
The approach is similar for both assay and impurity tests:
7.1.2.1 Impurities are available
For the assay, this should involve demonstration of the discrimination of the analyte in the presence of impurities and/or excipients; practically, this can be done by spiking pure substances (drug substance or drug product) with appropriate levels of impurities and/or excipients and demonstrating that the assay result is unaffected by the presence of these materials (by comparison with the assay result obtained on unspiked samples).
For the impurity test, the discrimination may be established by spiking drug substance or drug product with appropriate levels of impurities and demonstrating the separation of these impurities individually and/or from other components in the sample matrix.
7.1.2.2 Impurities are not available
If impurity or degradation product standards are unavailable, specificity may be demonstrated by comparing the test results of samples containing impurities or degradation products to a second well-characterized procedure e.g.: pharmacopoeial method or other validated analytical procedure (independent procedure).As appropriate, this should include samples stored under relevant stress conditions: light, heat, humidity, acid/base hydrolysis and oxidation.
- for the assay, the two results should be compared;
- for the impurity tests, the impurity profiles should be compared.
Peak purity tests may be useful to show that the analyte chromatographic peak is not attributable to more than one component (e.g., diode array, mass spectrometry).
7.2.Linearity
A linear relationship should be evaluated across the range of the analytical procedure. It may be demonstrated directly on the drug substance (by dilution of a standard stock solution) and/or separate weighings of synthetic mixtures of the drug product components, using the proposed procedure. The latter aspect can be studied during investigation of the range.
Linearity should be evaluated by visual inspection of a plot of signals as a function of analyte concentration or content. If there is a linear relationship, test results should be evaluated by appropriate statistical methods, for example, by calculation of a regression line by the method of least squares. In some cases, to obtain linearity between assays and sample concentrations, the test data may need to be subjected to a mathematical transformation prior to the regression analysis. Data from the regression line itself may be helpful to provide mathematical estimates of the degree of linearity.
The correlation coefficient, y-intercept, slope of the regression line and residual sum of squares should be submitted. A plot of the data should be included. In addition, an analysis of the deviation of the actual data points from the regression line may also be helpful for evaluating linearity.
Some analytical procedures, such as immunoassays, do not demonstrate linearity after any transformation. In this case, the analytical response should be described by an appropriate function of the concentration (amount) of an analyte in a sample.
For the establishment of linearity, a minimum of 5 concentrations is recommended. Other approaches should be justified. 8
Procedure Prepare by dilution from one solution five standard solutions with concentrations between 0 and 200% (e.g., 20%, 60%, 100%, 140%, 180%) of the expected sample concentration. Each solution must be tested at least twice.
Plot the average response against the quantity (or concentration) of the component and use linear regression analysis to calculate the calibration line. If an internal standard is used, the linearity of its curve has to be determined similarly5
7.3 RANGE
The specified range is normally derived from linearity studies and depends on the intended application of the procedure. It is established by confirming that the analytical procedure provides an acceptable degree of linearity, accuracy and precision when applied to samples containing amounts of analyte within or at the extremes of the specified range of the analytical procedure.
The following minimum specified ranges should be considered:
- for the assay of a drug substance or a finished (drug) product: normally from 80 to 120 percent of the test concentration;
- for content uniformity, covering a minimum of 70 to 130 percent of the test concentration, unless a wider more appropriate range, based on the nature of the dosage form (e.g., metered dose inhalers), is justified;
- for dissolution testing: +/-20 % over the specified range;
e.g., if the specifications for a controlled released product cover a region from 20%, after 1 hour, up to 90%, after 24 hours, the validated range would be 0-110% of the label claim.
- for the determination of an impurity: from the reporting level of an impurity to 120% of the specification;
- for impurities known to be unusually potent or to produce toxic or unexpected pharmacological effects, the detection/quantitation limit should be commensurate with the level at which the impurities must be controlled;
Note:for validation of impurity test procedures carried out during development, it may be necessary to consider the range around a suggested (probable) limit.
- if assay and purity are performed together as one test and only a 100% standard is used, linearity should cover the range from the reporting level of the impurities to 120% of the assay specification.
7.4.Accuracy
Accuracy should be established across the specified range of the analytical procedure.
7.4.1. Assay
7.4.1.1 Drug Substance
Several methods of determining accuracy are available:
a) application of an analytical procedure to an analyte of known purity (e.g. reference material);
b) comparison of the results of the proposed analytical procedure with those of a second well-characterized procedure, the accuracy of which is stated and/or defined
c) accuracy may be inferred once precision, linearity and specificity have been established.
7.4.1.2 Drug Product
Several methods for determining accuracy are available:
a) application of the analytical procedure to synthetic mixtures of the drug product components to which known quantities of the drug substance to be analysed have been added;
b) in cases where it is impossible to obtain samples of all drug product components , it may be acceptable either to add known quantities of the analyte to the drug product or to compare the results obtained from a second, well characterized procedure, the accuracy of which is stated and/or defined
c) accuracy may be inferred once precision, linearity and specificity have been established.
7.4.2. Impurities (Quantitation)
Accuracy should be assessed on samples (drug substance/drug product) spiked with known amounts of impurities.
In cases where it is impossible to obtain samples of certain impurities and/or degradation products, it is considered acceptable to compare results obtained by an independent procedure The response factor of the drug substance can be used.
It should be clear how the individual or total impurities are to be determined e.g., weight/weight or area percent, in all cases with respect to the major analyte.
7.4.3. Recommended Data
Accuracy should be assessed using a minimum of 9 determinations over a minimum of 3 concentration levels covering the specified range (e.g., 3 concentrations/3 replicates each of the total analytical procedure).
Accuracy should be reported as percent recovery by the assay of known added amount of analyte in the sample or as the difference between the mean and the accepted true value together with the confidence intervals.
7.5 Precision
Validation of tests for assay and for quantitative determination of impurities includes an investigation of precision.
7.5.1. Repeatability
Repeatability should be assessed using:
a) a minimum of 9 determinations covering the specified range for the procedure (e.g., 3 concentrations/3 replicates each);
or
b) a minimum of 6 determinations at 100% of the test concentration.
7.5.2. Intermediate Precision
The extent to which intermediate precision should be established depends on the circumstances under which the procedure is intended to be used. The applicant shouldestablish the effects of random events on the precision of the analytical procedure. Typical variations to be studied include days, analysts, equipment, etc. It is not considered necessary to study these effects individually. The use of an experimental design (matrix) is encouraged.
7.5.3. Reproducibility
Reproducibility is assessed by means of an inter-laboratory trial. Reproducibility should be considered in case of the standardization of an analytical procedure, for instance, for inclusion of procedures in pharmacopoeias. These data are not part of the marketing authorization dossier.
7.5.4. Recommended Data
The standard deviation, relative standard deviation (coefficient of variation) and confidence interval should be reported for each type of precision investigated.
7.6.Detection Limit
Several approaches for determining the detection limit are possible, depending on whether the procedure is a non-instrumental or instrumental. Approaches other than those listed below may be acceptable.
7.6.1. Based on Visual Evaluation
Visual evaluation may be used for non-instrumental methods but may also be used with instrumental methods.
The detection limit is determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte can be reliably detected.
7.6.2. Based on Signal-to-Noise
This approach can only be applied to analytical procedures which exhibit baseline noise.
Determination of the signal-to-noise ratio is performed by comparing measured signals from samples with known low concentrations of analyte with those of blank samples and establishing the minimum concentration at which the analyte can be reliably detected. A signal-to-noise ratio between 3 or 2:1 is generally considered acceptable for estimating the detection limit.
7.6.3 Based on the Standard Deviation of the Response and the Slope
The detection limit (DL) may be expressed as:
DL = 3.3 σ/S
where σ= the standard deviation of the response
S = the slope of the calibration curve
The slope S may be estimated from the calibration curve of the analyte. The estimate of σmay be carried out in a variety of ways, for example:
7.6.3.1 Based on the Standard Deviation of the Blank
Measurement of the magnitude of analytical background response is performed by analyzing an appropriate number of blank samples and calculating the standard deviation of these responses.
7.6.3.2 Based on the Calibration Curve
A specific calibration curve should be studied using samples containing an analyte in the range of DL. The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation.
7.6.4 Recommended Data
The detection limit and the method used for determining the detection limit should be presented. If DL is determined based on visual evaluation or based on signal to noise ratio, the presentation of the relevant chromatograms is considered acceptable for justification.
In cases where an estimated value for the detection limit is obtained by calculation or extrapolation, this estimate may subsequently be validated by the independent analysis of a suitable number of samples known to be near or prepared at the detection limit.
7.7.Quantitation Limit
Several approaches for determining the quantitation limit are possible, depending on whether the procedure is a non-instrumental or instrumental. Approaches other than those listed below may be acceptable.
7.7.1. Based on Visual Evaluation
Visual evaluation may be used for non-instrumental methods but may also be used with instrumental methods.
The quantitation limit is generally determined by the analysis of samples with known concentrations of analyte and by establishing the minimum level at which the analyte can be quantified with acceptable accuracy and precision.
7.7.2. Based on Signal-to-Noise Approach
This approach can only be applied to analytical procedures that exhibit baseline noise.
Determination of the signal-to-noise ratio is performed by comparing measured signals from samples with known low concentrations of analyte with those of blank samples and by establishing the minimum concentration at which the analyte can be reliably quantified. A typical signal-to-noise ratio is 10:1.
7.7.3. Based on the Standard Deviation of the Response and the Slope
The quantitation limit (QL) may be expressed as:
QL = 10 σ/S
where σ= the standard deviation of the response
S = the slope of the calibration curve
The slope S may be estimated from the calibration curve of the analyte. The estimate of σmay be carried out in a variety of ways for example:
7.7.3.1 Based on Standard Deviation of the Blank
Measurement of the magnitude of analytical background response is performed by analyzing an appropriate number of blank samples and calculating the standard deviation of these responses.
7.7.3.2 Based on the Calibration Curve
A specific calibration curve should be studied using samples, containing an analyte in the range of QL. The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation.
7.7.4 Recommended Data
The quantitation limit and the method used for determining the quantitation limit should be presented
The limit should be subsequently validated by the analysis of a suitable number of samples known to be near or prepared at the quantitation limit.
7.8.Robustness
The evaluation of robustness should be considered during the development phase and depends on the type of procedure under study. It should show the reliability of an analysis with respect to deliberate variations in method parameters.
If measurements are susceptible to variations in analytical conditions, the analytical conditions should be suitably controlled or a precautionary statement should be included in the procedure. One consequence of the evaluation of robustness should be that a series of system suitability parameters (e.g., resolution test) is established to ensure that the validity of the analytical procedure is maintained whenever used.
In the case of liquid chromatography, examples of typical variations are:
- influence of variations of pH in a mobile phase;
- influence of variations in mobile phase composition;
- different columns (different lots and/or suppliers);
- temperature
In the case of gas-chromatography, examples of typical variations are:
- different columns (different lots and/or suppliers);
- temperature;
- flow rate.
7.9 System Suitability Testing
System suitability testing is an integral part of many analytical procedures. The tests are based on the concept that the equipment, electronics, analytical operations and samples to be analyzed constitute an integral system that can be evaluated as such. System suitability test parameters to be established for a particular procedure depend on the type of procedure being validated. 8
In the final analysis,purpose of validating method is to ensure the procurement of high quality data. After all, if the quality of data questionable, no meaningful conclusions can be reached about the quality of the product – which will have seriously determinal effects on stability data reviews, process validation reviews. Time invested in validating analytical methods in beginning pays big dividend in long run 10
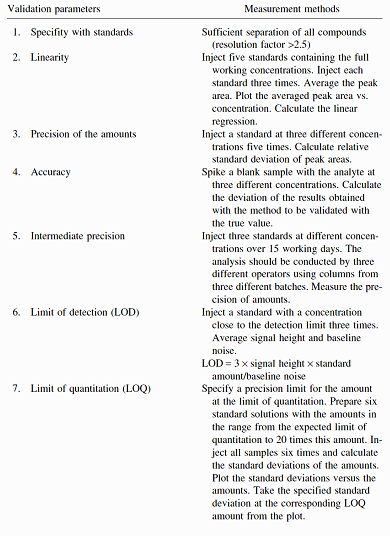
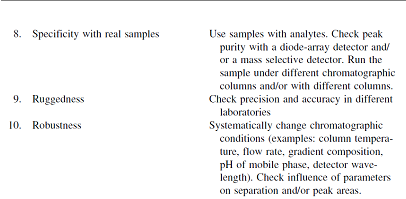
Table II:- Proposed Sequence of Validation Experiments, Example High Performance Liquid Chromatography 6
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
8. COMPARISON
8.1 According to USP
The performance characteristics required can be referred to as “Eight steps of Method Validation”. These analytical performance characteristics are
1. Accuracy
2. Precision
3. Specificity
4. Detection limit
5. Quantiation limit
6. Linearity and range
7. Ruggedness
8. Robustness
8.2 According to ICH
1. Accuracy
2. Precision
3. Repeatability
4. Intermediate precision
5. Specificity
6. Detection limit
7. Quantiation limit
8. Linearity
9. Range
10. Robustness
11. System suitability
8.3 According to FDA Reviewer Guidance
1. Accuracy
2. Precision
3. Repeatability
4. Injection repeatability
5. Analysis repeatibility
6. Intermediate precision
7. Specificity / selectivity
8. Reproducibility
9. Detection and quantiation limit
10. Quantiation limit
11. Linearity
12. Range
13. Robustness
14. System suitability specifications and tests
15. Sample solution stability 2
8.4 According to European guidelines
1. Accuracy
2. Precision
3. Repeatability
4. Intermediate precision
5. Specificity
6. Detection limit
7. Quantiation limit
8. Linearity
9. Range 3
Table III - USP data elements required for Validation13
|
Analytical performance parameters |
Assay category I |
Assay category II Quantitative Limit tests |
Assay category III |
Assay category IV |
|
|
Accuracy |
Yes |
Yes |
* |
* |
No |
|
Precision |
Yes |
Yes |
No |
Yes |
No |
|
Specificity |
Yes |
Yes |
Yes |
* |
Yes |
|
DL |
No |
No |
Yes |
* |
No |
|
QL |
No |
Yes |
No |
* |
No |
|
Linearity |
Yes |
Yes |
No |
* |
No |
|
Range |
Yes |
Yes |
* |
* |
No |
* indicates may be required on nature of specific test
Table IV - ICH elements required for Validation 2
|
Analytical performance parameters |
identification |
Impurity testing Quantitative Limit tests |
Assay |
|
|
Accuracy |
No |
Yes |
No |
Yes |
|
Precision |
|
|||
|
Repeatability |
No |
Yes |
No |
Yes |
|
Intermediate precision |
No |
Yes |
No |
Yes |
|
Specificity |
Yes |
Yes |
Yes |
Yes |
|
LOD |
No |
Yes |
Yes |
No |
|
LOQ |
No |
Yes |
No |
No |
|
Linearity |
Yes |
Yes |
No |
Yes |
|
Range |
Yes |
Yes |
No |
Yes |
Table V Japanese Pharmacopoeia elements required for Validation14
|
Validation characteristics/ type Of test |
Identification |
Impurity testing Quantitative Limit tests |
Assay |
|
|
Accuracy |
No |
Yes |
No |
Yes |
|
Precision |
|
|||
|
Repeatability |
No |
Yes |
No |
Yes |
|
Intermediate precision |
No |
No* |
No |
No* |
|
Reproducibility |
No |
No* |
No |
Yes* |
|
Specificity |
Yes |
Yes |
Yes |
Yes |
|
LOD |
No |
No |
Yes |
No |
|
LOQ |
No |
Yes |
No |
No |
|
Linearity |
No |
Yes |
No |
Yes |
|
Range |
No |
Yes |
No |
Yes |
* either intermediate precision or reproducibility should be evaluated depending upon circumstances in which analytical tests are performed
Table VI - Comparison of Analytical Parameters required for Validation 2
|
USP General chapter <1225> |
ICH Q2A Guidelines |
FDA Reviewer Guidance |
|
Accuracy |
Accuracy |
Accuracy |
|
Precision |
Precision |
Precision |
|
No |
Repeatability |
Repeatability Injection analysis |
|
No |
Intermediate precision |
Intermediate precision |
|
No |
No |
Reproducibility |
|
Specificity |
Specificity |
Specificity/selectivity |
|
Detection limit |
Detection limit
|
Detection limit
|
|
Quantiation limit |
Quantiation limit |
Quantiation limit |
|
Linearity
|
Linearity
|
Linearity
|
|
Ruggedness |
No |
No |
|
Robustness |
Robustness |
Robustness |
|
System suitability (discussed separately in USP 23 general chapter <621> |
System suitability
|
System suitability Sample solution stability
|
Conclusion
This article summarizes the validation parameters that are required according to the requirements of ICH Q2R(1). The paradigm shift under cGMP in the twenty - first century that requires the bench - level scientist to have the scientific and technical Understanding, product knowledge, process knowledge, and/or risk assessment abilities to appropriately execute the quality functions of analytical method validation In this article the method validation process and the minimum requirements to be included in a regulatory method are also discussed. Also a comparison between various parameters have been made i.e. in ICH, Pharmacopoeia & FDA and a table has been drawn from those observations
REFERENCES
1. Analytical Method Validation: Principles and Practices Available from URL http://www.pharmaquality.com/Media/.../Pgs28-31_ChapterPDF.pdf (Accessed on 06-August-2011)
2. Carstensen T. Jens, Rhodes C.T. “Drug Stability Principles and Practices” Third edition revised and expanded 2004, Marcel Dekker inc. P.NO.- 353-360
3. European network of official medicines control laboratories “Validation of Analytical Procedures”, june 2005 Available from URL http:// www.european-accreditation.org/.../00400OMCL%2005%2047%20 DEF3 (Accessed on 08-August-2011)
4. Guidance for Industry Q2B Validation of Analytical Procedures : Methodology, Nov.1996 Available from URL http:// www.fda.gov/downloads/. ../Guidances/ UCM 128049.pdf (Accessed on 25-July-2011)
5. Haider Imtiaz Syed “ Validating Standard Operating Procedure” second edition 2008 published by Informa Healthcare P.No.-448-460
6. Huber Ludwig “Validation of Analytical methods and processes” “ Pharmaceutical process Validation” 3rd edition revised & expanded 2003 Vol. 129 .Marcel dekker inc.
7. Huber Ludwig “Validation of Analytical Methods” A Primer, 2007 Published by Agilent Technologies Available from URL http:// www.chem.agilent .com/Library /primers/Public/5990-5140EN.pdf P.NO- 4(Accessed on 09-August-2011)
8. International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human use.”Validation of Analytical Procedures: Text and Methodology.ICH Q2(R1). Geneva(Nov. 2005) (Accessed on 25-July-2011)
9. Nash A. Robert “Validation of Analytical Procedure” “Encyclopedia of Pharmaceutical Technology” 3rd edition, 2003 volume 1, edited by James Swarbrick Informa healthcare Inc. P.NO – 3928-3929
10. Sarrio V. Robert, Silvestri J. Loui “ Validation of Analytical Assays and test methods for the pharmaceutical Laboratory”,1996 Available from URL http://www.regulatory.com/forum/article/test-val.html(Accessed on 26-July-2011)
11. Sharma P.P “Validation in Pharmaceutical Industry- Concept, Approaches & Guidelines, First Edition 2007 Published by Vandana Publications Pvt. Ltd. P.NO 361-362
12. Sharma P.P. “How to Practice GLP”, Second edition 2008 Published by Vandana Publications Pvt. Ltd. P.NO.-361-362
13. U.S. Pharmacopoeia (USP) 2007 General Chapter <1225> “Validation of Compendial Procedures”, USP, Rockville, MD P.NO..-2748-2749
14. Validation of Analytical Procedures 1330/ General Information JP XIV Available from URL http:// lib.njutcm.edu.cn/yaodian /jp/14data/ Validation_ of_Analytical _Pr.pdf (Accessed on 10-August-2011)
15. Validation of Analytical Procedures: Definition and Terminology, October 1998 (at step 7 for implementation) VICH (International cooperation on harmonisation of technical requirements for registration of veterinary medicinal plants) P.NO – 3 Available from URL http:// www.fda.gov/ downloads/ Animal Veterinary/ .. ./UCM052377.pdf (Accessed on 09-August-2011)
16. WHO Expert committee on specifications for pharmaceutical preparations, thirty second report world health organization geneva 1992 Available from URL http:// www.who.int/prequal/info_general/.../ WHO_TRS_929 _a nnex5FDCs.pdf P.NO. 4 (Accessed on 06-August-2011)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

