 ABOUT AUTHORS:
ABOUT AUTHORS:
Hiral H. Patel*, Paresh U. Patel,
Department of Pharmaceutical Analysis, Center for Health and Science Studies,
Ganpat University, Ganpat Vidyanagar – 384012,
Mehsana, Gujarat, India.
*patel.hiral2210@gmail.com
ABSTRACT
This method describes simple, sensitive, rapid, accurate, precise and economical derivative spectroscopic methodfor the simultaneous determination of tolperisone hydrochloride (TOL) and diclofenac sodium (DIC) in bulk and synthetic mixture. In this study, dual wavelength spectroscopic method was used for simultaneous determination of tolperisone hydrochloride and diclofenac sodium using the absorbance difference at two wavelengths. The absorbance differences of 257 nm and 306.20 nm were selected for the estimation of TOL and the absorbance differences of 243.40 nm and 265.40 nm were selected for estimation of DIC. The method was found to be linear (r2>0.999) in the range of 2- 18 μg/ml for tolperisone hydrochloride. The linear correlation was obtained (r2>0.997) in the range of 2- 18 μg/ml for diclofenac sodium. The limit of determination was 0.66 and 0.27 μg/ml for tolperisone hydrochloride and diclofenac sodium respectively. The limit of quantification was 2.00 and 0.83 μg/ml. The method was successfully applied for simultaneous determination of tolperisone hydrochloride and diclofenac sodium in binary mixture.
REFERENCE ID: PHARMATUTOR-ART-1696
INTRODUCTION
Tolperisone (TOL) is chemically 2-methyl-1-(4-methylphenyl)-3-(1-piperidyl) propan-1-one (Figure 1) is a well known antispasmodic drug1. It is official in Japanese Pharmacopoeia (JP). JP2 describe potentiometry method for its estimation. Literature survey reveals HPLC3 and UV4 method for estimation of TOL alone. Literature survey also reveals HPLC5 and UV spectrophotometry6 methods for determination of TOL with other drugs in combination. Diclofenac sodium (DIC) is chemically 2-[2,6diclohlorophenylamino] benzene acetic acid sodium salt7 (Figure 2). Diclofenac sodium (DIC) is official in Indian Pharmacopoeia (IP)and British Pharmacopoeia (BP). IP8 and BP9 describe liquid chromatography method for its estimation. Literature survey reveals HPLC10, 11 and UV12 method for determination of DIC alone. Literature survey also reveals HPLC13, 14, 15, UV spectrophotometry16 and HPTLC17 method for the determination of DIC with other drugs combination. The combination of these two drugs is not official in any pharmacopoeia; hence no official method is available for the simultaneous estimation of TOL and DIC in their combined synthetic mixture or dosage forms. Literature survey does not reveal any simple spectrophotometric method for simultaneous estimation of TOL and DIC in synthetic mixture or combined dosage forms. The present communication describes simple, sensitive, rapid, accurate, precise and cost effective spectrophotometric method based on derivative spectroscopic method for simultaneous estimation of both drugs in bulk and combined synthetic mixture.

MATERIALS AND METHODS
Apparatus
A shimadzu model 1700 (Japan) double beam UV/Visible spectrophotometer with spectral width of 2 nm, wavelength accuracy of 0.5 nm and a pair of 10 mm matched quartz cell was used to measure absorbance of all the solutions. Spectra were automatically obtained by UV-Probe system software. A Sartorius CP224S analytical balance (Gottingen, Germany), an ultrasonic bath (Frontline FS 4, Mumbai, India) was used in the study.
Reagents and materials
TOL and DIC bulk powder was kindly gifted by Torrent Research Centre, Gandhinagar, India and Acme Pharmaceuticals Ltd. Ahmadabad, Mehsana, Gujarat, India, respectively. Methanol (AR Grade, S. D. Fine Chemicals Ltd., Mumbai, India) and Whatman filter paper no. 41 (Millipore, USA) were used in the study.
Preparation of standard stock solutions
An accurately weighed standard TOL and DIC powder (10 mg) were weighed and transferred to 100 ml separate volumetric flasks and dissolved in methanol. The flasks were shaken and volumes were made up to mark with methanol to give a solution containing 100 μg/ml of each TOL and DIC.
Selection of wavelength
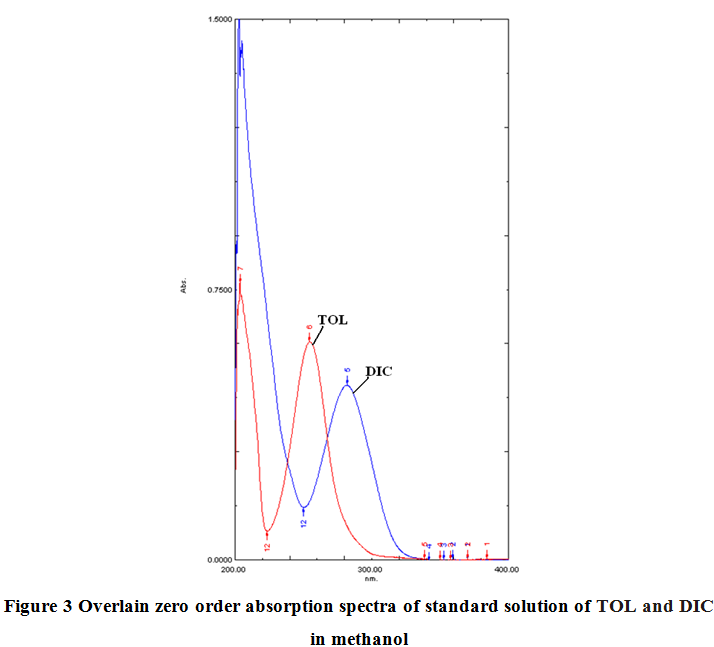
The standard solutions of TOL (10 µg/ml) and DIC (10 µg/ml) were scanned separately in the UV range of 200-400 nm. It appeared that at 257 nm and 306.20 nm DIC shows same absorbance so the absorbance differences of these wavelengths were selected for the estimation of TOL. While at 243.40 nm and 265.40 nm TOL shows same absorbance so the absorbance differences of these wavelengths were selected for estimation of DIC.
Validation of the proposed method
The proposed method was validated according to the International Conference on Harmonization (ICH) guidelines18.
Linearity (calibration curve)
To check linearity of the method, working standard solution having concentration in range of 2-18 µg/ml for TOL and 2-18 µg/ml for DIC were prepared from the standard stock solutions of both drugs. Aliquots of standard solution of TOL (0.2, 0.4, 0.6, 0.8, 1.0, 1.4, 1.8 ml) and DIC (0.2, 0.4, 0.6, 0.8, 1.0, 1.4, 1.8 ml) of standard stock solutions of both drug were transferred separately to a series of 10 ml volumetric flasks and diluted to mark with methanol, and the absorbance difference of 257 nm and 306.20 nm was measured for TOL and the absorbance difference of 243.40 nm and 265.40 nm was measured for DIC. The calibration curves were constructed by plotting absorbance difference vs. concentration.
Method precision (repeatability)
The precision of the instrument was checked by repeated scanning and measuring the absorbance of solution of (n = 6) TOLand DIC (8 µg/ml) without changing the parameters of First Derivative Method.
Intermediate precision (reproducibility)
The intraday and interday precision of the proposed method was determined by analyzing the corresponding responses 3 times on the same day and on 3 different days over a period of 1 week for 3 different concentrations of standard solutions of TOL and DIC (4, 8, 12 µg/ml for TOL and 4, 8, 12 µg/ml for DIC). The result was reported in terms of relative standard deviation (% RSD).
Accuracy (recovery study)
The accuracy of the method was determined by calculating recovery of TOL and DIC by the standard addition method. Known amounts of standard solutions of TOL and DIC were added at 50, 100 and 150 % level to prequantified sample solutions of TOL and DIC (4 µg/ml for TOL and 4 µg/ml for DIC). The amounts of TOL and DIC were estimated by applying obtained values to the regression equation of the calibration curve.
Limit of detection and Limit of quantification
The limit of detection (LOD) and the limit of quantification (LOQ) of the drug were derived by calculating the signal-to-noise ratio (S/N, i.e., 3.3 for LOD and 10 for LOQ) using the following equations designated by International Conference on Harmonization (ICH) guidelines18.
LOD = 3.3 × σ/S
LOQ = 10 × σ/S
Where, σ = the standard deviation of the response and S = slope of the calibration curve
Analysis of synthetic mixture
After proper dilution of prepared solution of synthetic mixture the absorbance of the sample solution was measured at 257 nm and 306.20 nm for quantitation of TOL and at 243.40 nm and 265.40 nm for quantitation of DIC, respectively. The amounts of the TOL and DIC present in the sample solution were calculated by fitting the responses into the regression equation for TOL and DIC in the First Derivative Method.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULTS AND DISCUSSION
In dual wavelength method, the primary requirement for developing a method for analysis is that the entire spectra should follow the beer’s law at all the wavelength, which was fulfilled in case of both these drugs. In this Dual Wavelength Spectrophotometry method the linearity was observed in the concentration range of 2-18 µg/ml with co-efficient of correlation, (r2) = 0.999 for TOL by absorbance difference at 257 nm and 306.20 nm. For DIC the linearity was observed in the concentration range of 2-18 µg/ml with co-efficient of correlation, (r2) = 0.997 by absorbance difference at 243.40 nm and 265.40 nm.
The RSD values of TOL were found to be 0.22 %. The RSD value of DIC was found to be 0.92 % . Relative standard deviation was less than 2 %, which indicates that proposed method is repeatable. The low RSD values of interday (0.22-0.42 % for TOL and 0.26-0.86 % for DIC) and intraday (0.15 – 0.67 % for TOL and 0.16 – 0.85 % forDIC) variation for TOL and DIC, reveal that the proposed method is precise. LOD and LOQ values for TOL were found to be 0.66 and 2.00 µg/ml, respectively. LOD and LOQ values for DIC were found to be 0.27 and 0.83 µg/ml respectively. These data show that method is sensitive for the determination of TOL and DIC. The regression analysis data and summary of validation parameters for the proposed method is summarized in Table 1.
The recovery experiment was performed by the standard addition method. The mean recoveries were 99.68 ± 0.31 and 99.67 ± 0.39 %for TOL and DIC, respectively (Table 2). The resultsof recovery studies in DIC state that the proposed method is highly accurate. The proposed validated method was successfully applied to determine TOL and DIC in their combined dosage form. The results obtained for TOL and DIC were comparable with the corresponding labeled amounts (Table 3). No interference of the excipients with the absorbance of interest appeared; hence the proposed method is applicable for the routine simultaneous estimation of TOL and DIC in pharmaceutical dosage forms.
Table 1: Regression analysis data and summary of validation parameters for the proposed method
|
PARAMETERS |
TOL at 250.60nm |
DIC at 311.20nm |
|
Concentration range (µg/ml) |
2-18 |
2-18 |
|
Slope |
0.061 |
0.001 |
|
Intercept |
0.013 |
0.007 |
|
Correlation coefficient |
0.999 |
0.997 |
|
LOD (µg/ml) |
0.66 |
0.27 |
|
LOQ (µg/ml) |
2.00 |
0.83 |
|
Repeatability (RSD, n = 6), % |
0.2199 |
0.9245 |
|
Precision (RSD), % |
|
|
|
Interday (n = 6),% Intraday (n = 6), % |
0.22-0.42 0.15 – 0.67 |
0.26-0.86 0.16 – 0.85 |
|
Recovery |
99.68 ± 0.31 |
99.67 ± 0.39 |
aRSD = Relative standard deviation. bLOD = Limit of detection. cLOQ = Limit of quantification. dS. D. is standard deviation
Table 2: Recovery data of proposed method
|
Drug |
Level |
Amount of sample taken (µg/ml) |
Amount of standard spiked (%) |
Mean % Recovery ± SD* |
|
TOL |
I |
10 |
50 %
|
99.50 ± 0.45 |
|
II |
10 |
100 %
|
100.23 ± 0.10 |
|
|
III |
10 |
150 % |
99.31 ± 0.36 |
|
|
DIC |
I |
10 |
50 %
|
100.21 ± 0.65 |
|
II |
10 |
100 %
|
99.40 ± 0.37 |
|
|
III |
10 |
150 % |
99.42 ± 0.15 |
Table 3: Analysis of TOL and DIC in synthetic mixture
|
Sample No. |
Amount Taken |
Amount Found |
% Assay |
|||
|
TOL (mg) |
DIC (mg) |
TOL (mg) |
DIC (mg) |
TOL % |
DIC % |
|
|
1 |
150 |
50 |
150.81 |
50.35 |
100.54 |
100.71 |
|
2 |
150 |
50 |
150.81 |
50.00 |
100.54 |
100.00 |
|
3 |
150 |
50 |
151.21 |
49.28 |
100.81 |
98.57 |
|
4 |
300 |
100 |
299.73 |
99.28 |
99.91 |
100.71 |
|
5 |
300 |
100 |
296.61 |
100.35 |
98.87 |
100.35 |
|
6 |
300 |
100 |
300.30 |
101.07 |
100.12 |
99.64 |
|
Mean |
100.13 |
99.99 |
||||
|
S.D. |
0.6991 |
0.8130 |
||||
CONCLUSION
The proposed Spectrophotometric method was found to be simple, sensitive, accurate and precise for determination of TOL and DIC in synthetic mixture. The method utilizes easily available and cheap solvent for analysis of TOL and DIC hence the method was also economic for estimation of TOL and DIC from synthetic mixture. The common excipients and additives are usually present in the synthetic mixture do not interfere in the analysis of TOL and DIC in method, hence it can be conveniently adopted for routine quality control analysis of the drugs in mixture or combined pharmaceutical formulation.
ACKNOWLEDGEMENT
The authors are thankful to Torrent Research Centre, Gandhinagar, India and Acme Pharmaceutical Ltd., Ahmadabad, India for providing gift sample of TOL and DIC, respectively for carry out the research work. The authors are highly thankful to Center for Health and Science Studies, Ganpat University, Ganpat Vidyanagar – 384012, Mehsana, Gujarat, India for providing all the facilities to carry out the research work.
REFERENCES
1. Maryadele. J. O’ Neil. The Merck Index: An Encyclopedia of chemicals, drugs and biologicals, 14th edition. New Jersey: Published by Merck Research Laboratories, Division of Merck and Co., Inc. Whitehouse station; 2006: 1698.
2. Japanese Pharmacopeia, 15th ed, Society of Japanese Pharmacopeia; 2006: 1190-1191.
3. Murali M, Satyanarayana PV. Simple validated isocratic RP-HPLC method for estimation of tolperisone in bulk and pharmaceutical dosage form. Der Pharm Chemica 2011; 3: 13-19.
4. Koladia BB, Vaghela VM. UV spectroscopic method for quantitative estimation of tolperisone hydrochloride in bulk and pharmaceutical dosage form. Int J PharmTech Res 2012; 4: 1317-22.
5. Liawruangrath S, Liawruangrath B, Pibool P. Simultaneous determination of tolperisone and lidocaine by HPLC. J Pharm Biomed Anal 2001; 26: 865-72.
6. Sharma KK, Patel PU. First derivative spectroscopic method for simultaneous estimation of paracetamol and tolperisone in their combined dosage form. J Pharm Sci Bio Res 2012; 2: 92-96.
7. Maryadele. J. O’ Neil. The Merck Index: An Encyclopedia of chemicals, drugs and biologicals, 14th edition. New Jersey: Published by Merck Research Laboratories, Division of Merck and Co., Inc. Whitehouse station; 2006: 542
8. Indian Pharmacopoeia, Vol. II, New Delhi, The Controller Publication, Govt. of India; 2010: 1199.
9. British Pharmacopoeia, Vol. I, London, The British Pharmacopoeia Commission; 2010: 672.
10. El-sayed YM, Abdel-hameed ME, Suleiman MS, Najib NM. A rapid and sensitive HPLC method for the determination of DICfenac sodium in serum. J Pharm Pra 2011; 40: 757-729.
11. Mayee R, Rawat S, Thosar A, Atre K, Mane P. Development and validation of HPLC method for determination of DICfenac sodium by tape stripping method. Asian J Pharm Bio Res. 2011; 1: 317-22.
12. Khaskheli AR, Abro K, Sherazi ST, Afridi HI, Mahesar SA, Saeed M. Simple and faster spectrophotometric determination of DICfenac sodium in tablet, serum and urine samples. Pak J Anal Environ Chem 2009; 10: 53-8
13. Gowramma B, Rajan S, Muralidharan S, Meyyanathan SN, Suresh B. Validated HPLC method for simultaneous estimation of paracetamol and DICfenac in pharmaceutical formulation. Int J ChemTech Res 2010; 2: 676-80.
14. Mulgund SV, Phoujdar MS, Londhe SV, Mallade PS, Kulkarni TS, Deshpande AS. Stability indicating HPLC method for simultaneous determination of mephenesin and DICfenac diethyl amine. Indian J Pharm Sci 2009; 71: 35-40.
15. Shinde VM, Desai BS. Simultaneous estimation of paracetamol, DICfenac and chlorzoxazone from tablet by HPLC. Indian J Pharm Sci 2008; 57: 35-7.
16. Revathi G, Rama Rao N, Venkata SP. Simultaneous UV spectrophotometric determination and validation of DICfenac sodium and rabeprazole sodium using hydrotropic agents in its tablet dosage form. Int J Drug Dev Res 2012; 4: 316-24.
17. Dhaneshwar SR, Bhusari VK. Validated HPTLC method for simultaneous quantitation of DICfenac sodium and misoprostol in bulk drug and formulation. Asian J Pharm Bio Res 2011; 1: 15-21.
18. The International Conference on Harmonization, Q2 (R1), Validation of Analytical Procedure, Text and Methodology, 2005.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE










.png)

