About Authors: Divya Gupta,
M.Pharm (Pharmaceutics),
Dehradun Institute of Technology, Dehradun
Introduction
In the words of Hughes and Mitra2: “ophthalmic drug delivery is one of the most interesting and challenging endeavours facing the pharmaceutical scientist...The anatomy, physiology and biochemistry of the eye render this organ exquisitely impervious to foreign substances...The challenge to the formulator is to circumvent the protective barriers of the eye without causing permanent tissue damage...The primitive ophthalmic solutions, suspensions and ointment dosage forms are clearly no longer sufficient to combat some present virulent diseases...”
Eye is a unique and very valuable organ. This is considered a window hinge. We can enjoy it and look at the world body. There are many eye diseases that can affect the body and loss of vision as well. Therefore, many eyes in drug delivery systems are available. They are classified as traditional and new drug development system. Topical application of drugs to the eye is the most popular and well-accepted route of administration for the treatment of various eye disorders. The bioavailability of ophthalmic drugs is, however, very poor due to efficient protective mechanisms of the eye. Blinking, baseline and reflex lachrymation, and drainage remove rapidly foreign substances, including drugs, from the surface of the eye [1].
There are many eye ailments which affected to eye and one can loss the eye sight also. Therefore many ophthalmic drug delivery systems are available. These are classified as conventional and non-conventional (newer) drug delivery systems. Most commonly available ophthalmic preparations are eye drops and ointments about 70% of the eye dosage formulations in market. But these preparations when instilled into the culde-sac are rapidly drained away from the ocular cavity due to tear flow and lachrymal nasal drainage. Only a small amount is available for its therapeutic effect resulting in frequent dosing. So overcome to these problems newer pharmaceutical ophthalmic formulation such as in-situ gel, nanoparticle, liposome, nanosuspension, microemulsion, intophoresis and ocular inserts have been developed in last three decades increase the bioavailability of the drug as a sustained and controlled manner [2-9].
Reference ID: PHARMATUTOR-ART-1094
Advantages of ocular drug delivery systems
1. Increased accurate dosing. To overcome the side effects of pulsed dosing produced by conventional systems.
2. To provide sustained and controlled drug delivery.
3. To increase the ocular bioavailability of drug by increasing the corneal contact time. This can be achieved by effective adherence to corneal surface.
4. To provide targeting within the ocular globe so as to prevent the loss to other ocular tissues.
5. To circumvent the protective barriers like drainage, lacrimation and conjunctival absorption.
6. To provide comfort, better compliance to the patient and to improve therapeutic performance of drug.
7. To provide better housing of delivery system.
Limitations of ophthalmic drug delivery:
1. Dosage form cannot be terminated during emergency.
2. Interference with vision.
3. Difficulty in placement and removal.
4. Occasional loss during sleep or while rubbing eyes.
Despite these limitations, significant improvements in ocular drug delivery have been made. The improvements have been with objective of maintaining the drug in the bio-phase for an extended period. The anatomy, physiology and biochemistry of the eye render this organ impervious to foreign substances [10].
Anatomy and function of the eye
The eye is a spherical structure with a wall consisting of three layers; the outer sclera, the middle choroid layer, Ciliary body and iris and the inner nervous tissue layer retina. The sclera is tough fibrous coating that protects the inner layers. It is white except for the transparent area at the front, the cornea which allow light to enter the eye. The choroid layer, situated inside the sclera, contains many blood vessels and is modified at the front of the eye as pigmented iris. The iris is the coloured part of the eye (in shades of blue, green, brown, hazel, or grey) [11].
The structure of the cornea
The cornea is a strong clear transparent bulge located at the front of the eye that conveys images to the back of the eyes. The front surface of the adult cornea has a radius of approximately 8mm that covers about one-sixth of the total surface of the eye ball. It is a vascular tissue to which nutrient and oxygen are supplied via bathing with lachrymal fluid and aqueous humour as well as from blood vessels that lines the junction between the cornea and sclera (in fig.1) [12].
The cornea is the main pathway permeation of drug into the eye. It is composed of five layers: epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium [13, 14]. The epithelium consists of 5 to 6 layers of cells. The corneal thickness is 0.5–0.7 mm and it is thicker in the central region. The corneal epithelium is the main barrier of drug absorption into the eye in Comparison to many other epithelial tissues (intestinal, nasal, bronchial, and tracheal) corneal epithelium is relatively impermeable [15]. The epithelium is squamous stratified, consisting of 5-6 layer of cells with a total thickness around 50-100 μm and turnover of about one cell layer per day. The basal cells are packed closely together with a tight junction, to forming not only an effective barrier to most microorganisms, but also for drug absorption. Drugs penetrate across the corneal epithelium via the transcellular or paracellular pathway. Lipophilic drugs prefer the transcellular route and hydrophilic drugs penetrate primarily through the paracellular pathway which involves passive or altered diffusion through intercellular spaces. For most topically applied drugs, passive diffusion along their concentration gradient, either transcellularly or paracellularly, is the main permeation mechanism across the cornea.
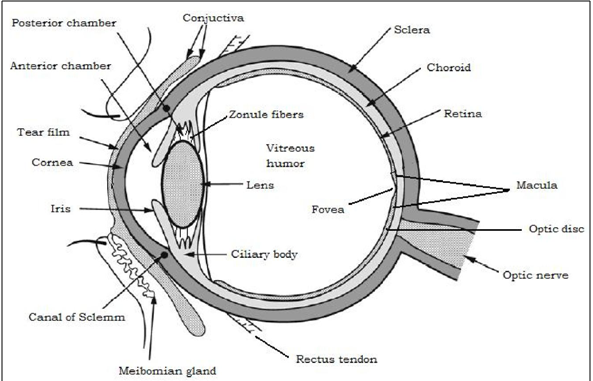
Figure1: Structure of the eye
The Bowman’s membrane is an acellular homogeneous sheet, about 8-14μm thick situated between the basement membrane of the epithelium and the stroma. The stroma, or substania propria, accounts for around 90% of the corneal thickness and contains approximately 85% water and about 200-250 collagenous lamellae. The lamellae provide physical strength while permitting optical transparency. The stroma has a relatively open structure and will normally allow the diffusion of hydrophilic solutes. The descemet’s membrane is secreted by the endothelium. It lies between the stroma and the endothelium [11, 12].
Conjunctiva
The conjunctiva is involved in the formation and maintenance of the precorneal tear film and in the protection of the eye. The conjunctiva is a thin transparent membrane, which lines the inner surface of the eyelids and is reflected onto the globe. The membrane is vascular and moistened by the tear film. The conjunctiva is composed of an epithelium, a highly vascularised substantia propria, and a submucosa or episclera. The bulbar epithelium consists of 5 to 7 cell layers. The structure resembles a palisade and not a pavement when compared to the corneal epithelium. At the surface, epithelial cells are connected by tight junctions, which render the conjunctiva relatively impermeable. The conjunctival tissue is permeable to molecules up to 20,000 Da, whereas the cornea is impermeable to molecules larger than 5000 Da. The human conjunctiva is between 2 and 30 times more permeable to drugs than the cornea and it has been proposed that loss by this route is a major path for drug clearance. There are 1.5 million globlet cell present in the conjunctiva with the highest density is in the Inferonasal quadrant (10 goblet cells/mm2). The highest density found in the children and adults varying with age depended among the intersujects variability. A significant increase in the number of goblet cells was reported in the case of vernal conjunctivitis and atopic kerato conjunctivitis but a great variation in goblet cell density results only in a small difference in tear mucin concentration [14, 15].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Nasolachrymal drainage system
Nasolachrymal drainage system consists of three parts; the secretory system, the distributive system and the excretory system. The secretory portion is composed of the lacrimal gland that secreted tears are spread over the ocular surface by the eyelids during blinking. The secretory system is stimulated by blinking and temperature change due to the tear evaporation and reflux secretors that have an efferent parasympathetic nerve supply and secrete in response to physical and emotional stimulation e.g. crying. The distributive system consists of the eyelids and the tear meniscus around the lid edges of the open eye, which spread tears over the ocular surface by blinking, thus preventing dry areas from developing. The excretory part of the Nasolachrymal drainage system consists of the lachrymal puncta, the superior, inferior and common canaliculi; the lachrymal sac, and the nasochrymal duct. In humans, the two puncta are the openings of the lachrymal canaliculi and are situated on an elevated area known as the lachrymal papilla. It is thought that tears are largely absorbed by the mucous membrane that lines the ducts andthe lachrymal sac; only a small amount reaches the nasal passage [11,14].
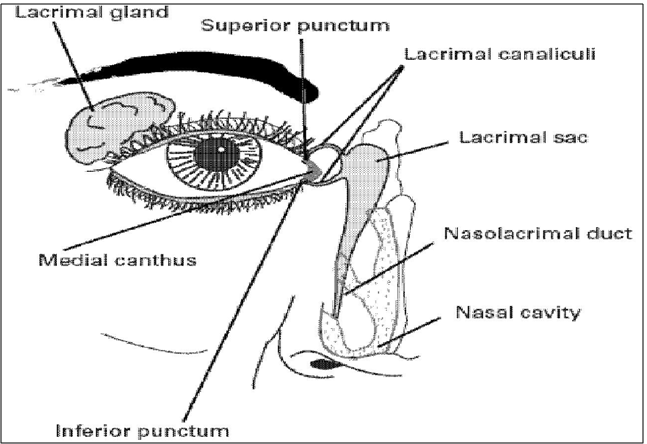
Figure 2: Schematic diagram of naso-lachrymation drainage system
Tear film
The exposed part of the eye is covered by a thin fluid layer, the so-called precorneal tear film. The film thickness is reported to be about 3–10 Am depending on the measurement method used. The resident volume amounts to about 10μl. The osmolality of the tear film equals 310–350 mOsm/kg in normal eyes and is adjusted by the monovalent and divalent inorganic ions such as Na+, K+, Cl-, HCO3-, and proteins. The mean pH value of normal tears is about 7.4. Diurnal patterns of pH changes exist, with a general shift from acid to alkaline during the day. The buffer capacity of the tears is determined by bicarbonate ions, proteins, and mucins [16, 17]. Tears exhibit a non- Newtonian rheological behaviour. The viscosity is about 3 mPas [13]. The mean surface tension value is about 44 mN/m.
Ocular pharmacokinetics
The main routes of drug administration and elimination from the eye have been shown schematically in Fig. 3.
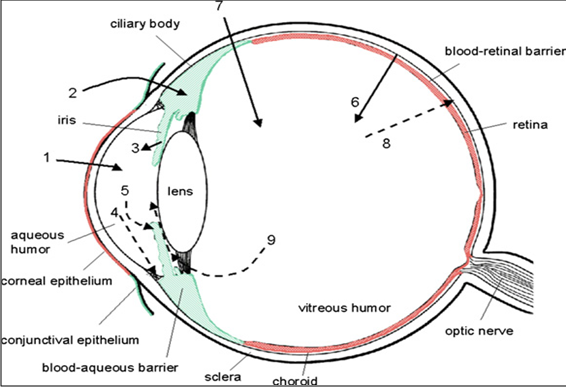
Fig. 3: Schematic presentation of the ocular structure with the routes of drug kinetics illustrated. The numbers refer to following processes: 1) transcorneal permeation from the lacrimal fluid into the anterior chamber, 2) non-corneal drug permeation across the conjunctiva and sclera into the anterior uvea, 3) drug distribution from the blood stream via blood-aqueous barrier into the anterior chamber, 4) elimination of drug from the anterior chamber by the aqueous humor turnover to the trabecular meshwork and Sclemm's canal, 5) drug elimination from the aqueous humor into the systemic circulation across the blood-aqueous barrier, 6) drug distribution from the blood into the posterior eye across the blood-retina barrier, 7) intravitreal drug administration, 8) drug elimination from the vitreous via posterior route across the blood-retina barrier, and 9) drug elimination from the vitreous via anterior route to the posterior chamber.
The barriers
Drug loss from the ocular surface
After instillation, the flow of lacrimal fluid removes instilled compounds from the surface of the eye. Even though the lacrimal turnover rate is only about 1 μl/min the excess volume of the instilled fluid is flown to the nasolacrimal duct rapidly in a couple of minutes [18]. Another source of non-productive drug removal is its systemic absorption instead of ocular absorption. Systemic absorption may take place either directly from the conjunctival sac via local blood capillaries or after the solution flow to the nasal cavity [19,20]. Anyway, most of small molecular weight drug dose is absorbed into systemic circulation rapidly in few minutes. This contrasts the low ocular bioavailability of less than 5% [18].
Drug absorption into the systemic circulation decreases the drug concentration in lacrimal fluid extensively. Therefore, constant drug release from solid delivery system to the tear fluid may lead only to ocular bioavailability of about 10%, since most of the drug is cleared by the local systemic absorption anyway [21].
Lacrimal fluid-eye barriers
Corneal epithelium limits drug absorption from the lacrimal fluid into the eye [22]. The corneal barrier is formed upon maturation of the epithelial cells. They migrate from the limbal region towards the centre of the cornea and to the apical surface. The most apical corneal epithelial cells form tight junctions that limit the paracellular drug permeation [23]. Therefore, lipophilic drugs have typically at least an order of magnitude higher permeability in the cornea than the hydrophilic drugs [24]. Despite the tightness of the corneal epithelial layer, transcorneal permeation is the main route of drug entrance from the lacrimal fluid to the aqueous humor (Fig. 3). In general, the conjunctiva is more leaky epithelium than the cornea and its surface area is also nearly 20 times greater than that of the cornea [25, 26]. Drug absorption across the bulbar conjunctiva has gained increasing attention recently, since conjunctiva is also fairly permeable to the hydrophilic and large molecules [27]. Therefore, it may serve as a route of absorption for larger bio-organic compounds such as proteins and peptides. Clinically used drugs are generally small and fairly lipophilic. Thus, the corneal route is currently dominating. In both membranes, cornea and conjunctiva, principles of passive diffusion have been extensively investigated, but the role of active transporters is only sparsely studied.
Blood-ocular barriers
The eye is protected from the xenobiotics in the blood stream by blood-ocular barriers. These barriers have two parts: blood-aqueous barrier and blood-retina barrier.
The anterior blood-eye barrier is composed of the endothelial cells in the uvea. This barrier prevents the access of plasma albumin into the aqueous humor, and limits also the access of hydrophilic drugs from plasma into the aqueous humor. Inflammation may disrupt the integrity of this barrier causing the unlimited drug distribution to the anterior chamber. In fact, the permeability of this barrier is poorly characterised. The posterior barrier between blood stream and eye is comprised of retinal pigment epithelium (RPE) and the tight walls of retinal capillaries [22,23]. Unlike retinal capillaries the vasculature of the choroid has extensive blood flow and leaky walls. Drugs easily gain access to the choroidal extravascular space, but thereafter distribution into the retina is limited by the RPE and retinal endothelia. Despite its high blood flow the choroidal blood flow constitutes only a minor fraction of the entire blood flow in the body. Therefore, without specific targeting systems only a minute fraction of the intravenous or oral drug dose gains access to the retina and choroid. Unlike blood brain barrier, the blood-eye barriers have not been characterised in terms of drug transporter and metabolic enzyme expression. From the pharmacokinetic perspective plenty of basic research is needed before the nature of blood-eye barriers is understood.
Corneal and Non-Corneal Routes of Absorption
Lacrimal drainage and systemic absorption from the conjunctiva can wash away ophthalmic drops which are the most common type of ocular drugs. This results in absorption of a small fraction of the drug. [28,29,30] For topical drugs, small lipophilic molecules are normally absorbed through the cornea, while large hydrophilic molecules such as proteins/gene based medicines are absorbed via the conjunctiva and sclera.[31] Of these routes, the mechanical and chemical barrier functions of the cornea control access of exogenous substances into the eye, thereby protecting intraocular tissues (Fig. 4).
The human cornea measures approximately 12 mm in diameter and 520 μm in thickness, and consists of five layers, including the epithelium, basement membrane (Bowman's layer), stroma, Descemet's membrane and endothelium (Fig. 3).
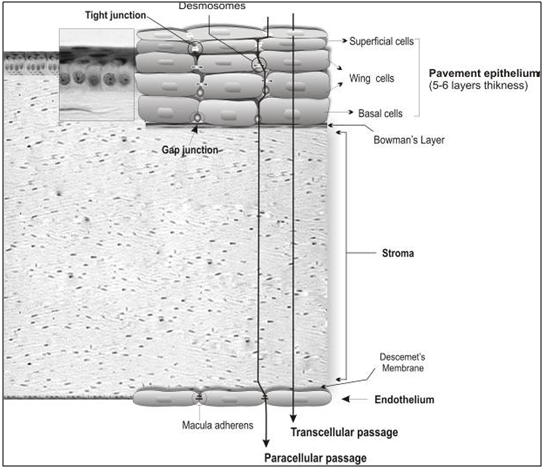
Figure 4: Corneal cellular organization, the cornea consists of various transport limiting layers. The tightest monolayer is made by outer superficial epithelial cells which display tight junction complexes. The wing and basal cells exhibit gap junctions. The stroma and Descemet’s membrane cover the inner endothelial cells which contain macula adherens and are more permeable.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
The human corneal epithelium is a stratified, squamous, non-keratinized epithelium 50 μm in thickness. It is composed of two to three layers of flattened superficial cells, wing cells, and a single layer of columnar basal cells which are separated by a 10–20 nm intercellular spaces and have regular intercommunications. These desmosome-attached cells can communicate via gap junctions through which small molecules traverse. Tight junctions (zonulae occludens) seal the superficial cells, building a diffusion barrier in the surface of the epithelium. Compared to the stroma and endothelium, the corneal epithelium represents a rate-limiting barrier which hinders permeation of hydrophilic drugs and macromolecules. The stroma displays hydrophilic nature due to an abundant content of hydrated collagen, which prevents diffusion of highly lipophilic agents. The corneal endothelial monolayer maintains an effective barrier between the stroma and aqueous humor. [32] Active ion and fluid transport mechanisms in the endothelium are responsible for maintaining corneal transparency. [33] It has been reported that certain drug properties such as lipophilicity, molecular weight, charge, and degree of ionization can significantly influence its passive permeability across the cornea. [34] Of these factors, lipophilicity plays a key role since transcellular permeation of lipophilic drugs through the cornea is faster and greater as compared to hydrophilic drugs. This route appears to be the main path for absorption of topical drugs. Greater molecular size decreases the rate of paracellular permeation of drugs. [35, 36] Once in the cornea, the drug can diffuse into the aqueous humor and the anterior segment (Fig. 3). However, local administration of conventional drugs via the corneal route fails to provide adequate concentrations within the vitreous and retina. [37,38] The conjunctiva is a mucous membrane consisting of vascularized epithelium (2-3 cell layers thick) and plays an important role as a protective barrier on the ocular surface since tight junctions are present on the apical surface of its cells. In fact, the bulbar conjunctiva represents the first barrier against permeation of topically applied drugs via the non-corneal route, which is the main intraocular route for entry of macromolecules and hydrophilic substances. Due to significant loss of drug through systemic circulation, the conjunctival sclera pathway appears to be a non-efficient path resulting in poor bioavailability. [39] The sclera is about 10 times more permeable than the cornea and half permeable as the conjunctiva. It is poorly vascularized and consists mainly of collagen and mucopolysaccharides, through which drugs can diffuse and enter the posterior segment (uveal tract, retina, choroid, vitreous humor).
Diffusion characteristics of various drugs were studied. Scleral permeability was significantly higher than that in cornea, and permeability coefficients of the beta-blockers ranked as follows: propranolol > penbutolol > timolol > nadolol for cornea, and penbutolol > propranolol > timolol > nadolol for the sclera.
Routes of ocular drug delivery
There are several possible routes of drug delivery into the ocular tissues (Fig. 3). The selection of the route of administration depends primarily on the target tissue. Traditionally topical ocular and subconjunctival administrations are used for anterior targets and intravitreal administration for posterior targets. Design of the dosage form can have big influence on the resulting drug concentrations and on the duration of drug action.
Topical ocular
Typically topical ocular drug administration is accomplished by eye drops, but they have only a short contact time on the eye surface. The contact, and thereby duration of drug action, can be prolonged by formulation design (e.g. gels, gelifying formulations, ointments, and inserts) [23]. During the short contact of drug on the corneal surface it partitions to the epithelium and in the case of lipophilic compounds it remains in the epithelium and is slowly released to the corneal stroma and further to the anterior chamber [40]. After eye drop administration the peak concentration in the anterior chamber is reached after 20–30 min, but this concentration is typically two orders of magnitude lower than the instilled concentration even for lipophilic compounds [21]. From the aqueous humor the drug has an easy access to the iris and ciliary body, where the drug may bind to melanin. Melanin bound drug may form a reservoir that is released gradually to the surrounding cells, thereby prolonging the drug activity. Distribution to the lens is much slower than the distribution to the uvea [22]. Unlike porous uvea, the lens is tightly packed protein rich structure where drug partitioning takes place slowly. Drug is eliminated from the aqueous humor by two main mechanisms: by aqueous turnover through the chamber angle and Sclemm's canal and by the venous blood flow of the anterior uvea [22] (Fig. 3). The first mechanism has a rate of about 3μl/min and this convective flow is independent of the drug. Elimination by the uveal blood flow, on the other hand, depends on the drug's ability to penetrate across the endothelial walls of the vessels. For this reason, clearance from the anterior chamber is faster for lipophilic than for hydrophilic drugs. Clearance of lipophilic drugs can be in the range of 20–30 μl/min. In those cases, most of drug elimination takes place via uveal blood flow. Halflifes of drugs in the anterior chamber are typically short, about an hour. The volumes of distribution are difficult to determine due to the slow equilibration of drug in the ocular tissues. The estimates in rabbits range from the volume of aqueous humor (250 μl) up to 2 ml [23]. In the latter case, the slow drug distribution to the vitreous is included in the volume of distribution. This distribution is slow, because the lens prohibits drug access to the vitreous. Flow of aqueous humor from the posterior chamber to the anterior chamber is another limiting factor. Some part of topically administered drugs may absorb across the bulbar conjunctiva to the sclera and further to the uvea and posterior segment (Fig. 3). This is an inefficient process, but may be improved by dosage forms that release drug constantly to the conjunctival surface. The role of this non-corneal route of absorption depends on the drug properties. Generally more hydrophilic and larger molecules may absorb via this route. They have particularly poor penetration across the cornea, and therefore, the relative contribution of the non-corneal is more eminent. Delivery across the conjunctiva and further to the posterior segment would be desirable, but unfortunately the penetration is clinically insignificant.
Sub-conjunctival administration.
Traditionally subconjunctival injections have been used to deliver drugs at increased levels to the uvea. Currently this mode of drug delivery has gained new momentum for various reasons. The progress in materials sciences and pharmaceutical formulation have provided new exciting possibilities to develop controlled release formulations to deliver drugs to the posterior segment and to guide the healing process after surgery (e.g. glaucoma surgery) [41]. Secondly, the development of new therapies for macular degeneration (antibodies, oligonucleotides) must be delivered to the retina and choroid [42, 43].
After subconjunctival injection drug must penetrate across sclera which is more permeable than the cornea. Interestingly the scleral permeability is not dependent on drug lipophilicity [44]. In this respect it clearly differs from the cornea and conjunctiva. Even more interesting is the surprisingly high permeability of sclera to the large molecules of even protein size [45]. Thus, it would seem feasible to deliver drugs across sclera to the choroid. However, delivery to the retina is more complicated, because in this case the drug must pass across the choroid and RPE. The role of blood flow is well characterise kinetically but the based on the existing information, there are good reasons to believe that drugs may be cleared significantly to the blood stream in the choroid. Pitkänen et al. showed recently that RPEis tighter barrier that sclera for the permeation of hydrophilic compounds [44]. In the case of small lipophilic drugs they have similar permeabilities. More complete understanding of the kinetics in sclera, choroid and RPE should help to develop medications with optimal activity in the selected posterior target tissues. Combination of the kinetic knowledge and cell selective targeting moieties offer very interesting possibilities.
Intravitreal administration.
Direct drug administration into the vitreous offers distinct advantage of more straightforward access to the vitreous and retina (Fig. 3). It should be noted, however, that delivery from the vitreous to the choroid is more complicated due to the hindrance by the RPE barrier. Small molecules are able to diffuse rapidly in the vitreous but the mobility of large molecules, particularly positively charged, is restricted [46]. Likewise, the mobility of the nanoparticles is highly dependent on the structure. In addition to the diffusive movement convection also plays a role [47]. The convection results from the eye movements.
After intravitreal injection the drug is eliminated by two main routes: anterior and posterior [22]. All compounds are able to use the anterior route. This means drug diffusion across the vitreous to the posterior chamber and, thereafter, elimination via aqueous turnover and uveal blood flow. Posterior elimination takes place by permeation across the posterior bloodeye barrier. This requires adequate passive permeability (i.e. small molecular size, lipophilicity) or active transport across these barriers. For these reasons, large molecular weight and water-solubility tend to prolong the half-life in the vitreous [22].
Drugs can be administered to the vitreous also in controlled release formulations (liposomes, microspheres, implants) to prolong the drug activity.
Mechanism of controlled sustained drug release into the eye
The corneal absorption represents the major mechanism of absorption for the most conventional ocular therapeutic entities.
Passive Diffusion is the major mechanism of absorption for nor?erodible ocular insert with dispersed drug. Controlled release can further regulated by gradual dissolution of solid dispersed drug within this matrix as a result of inward diffusion of aqueous solution.
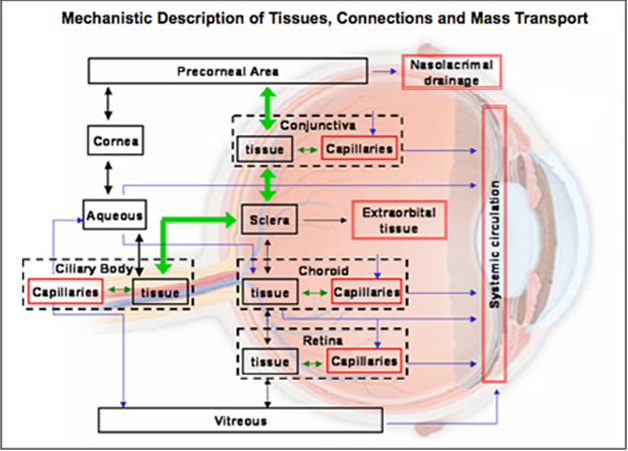
Corneal barrier limitation for topically administered drug
The existing ocular drug delivery systems are thus fair and in-efficient. The design of ocular system is undergoing gradual transition from an empirical to rational basis; Interest in the broad areas of ocular drug delivery has increased in recent years due to an increased understanding of a number of ocular physiological process and pathological conditions. The focus of this review is the approaches made towards optimization of ocular delivery systems
1. Improving ocular contact time
2. Enhancing corneal permeability
3. Enhancing site specificity [48]
Ophthalmic drug product may be classified according to route of administration.
1. Topical
2. Intraocular
3. Systemic (oral and venous).
Absorption of drugs in the eye takes place either through corneal or non?corneal route.
Maximum absorption takes place though the cornea, which leads the drug into aqueous humor. Loss of the administered dose of drug, takes place through spillage and removal by the naso?lacrimal apparatus. The non corneal route involves the absorption across the sclera and conjunctiva into the intra ocular tissues.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Drug Absorption and Disposition in the Eye
The pharmacokinetics and constraints of ocular drug absorption have been examined thoroughly in the literature. [49-58] A pharmacokinetic scheme illustrating the precorneal fluid dynamics and the distribution/ disposition of pilocarpine in rabbits is presented in Figure 5.
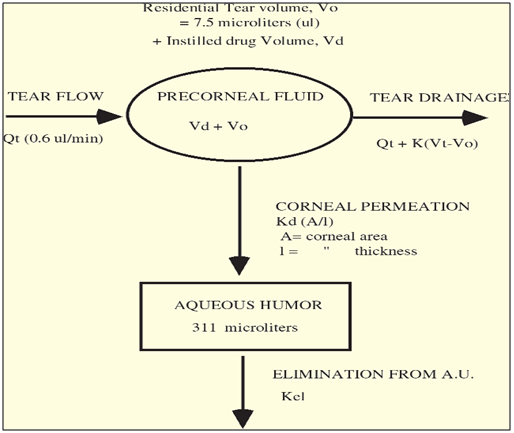
Figure 5: Pharmacokinetic Scheme Illustrating the Distribution of Pilocarpine from the Tear Fluid into the Aqueous Humour (modified from reference 59)
It is common knowledge that the ocular bioavailability of drugs applied topically as eye-drops is very poor. The absorption of drugs in the eye is severely limited by some protective mechanisms that ensure the proper functioning of the eye, and by other concomitant factors, for example:
• Drainage of the instilled solutions;
• Lacrimation and tear turnover;
• Metabolism;
• Tear evaporation;
• Non-productive absorption/adsorption;
• Limited corneal area and poor corneal permeability; and
• Binding by the lacrimal proteins.
The drainage of the administered dose via the nasolacrimal system into the nasopharynx and the gastrointestinal tract takes place when the volume of fluid in the eye exceeds the normal lacrimal volume of 7–10 microlitres. Thus, the portion of the instilled dose (one to two drops, corresponding to 50–100 microlitres) that is not eliminated by spillage from the palpebral fissure is quickly drained and the contact time of the dose with the absorbing surfaces (cornea and sclera) is reduced to a maximum of two minutes. The lacrimation and the physiological tear turnover (16% per minute in humans in normal conditions) can be stimulated and increased by the instillation even of mildly irritating solutions. The net result is a dilution of the applied medication and an acceleration of drug loss. It is now definitively established that the rate at which instilled solutions are removed from the eye varies linearly with instilled volume. In other words, the larger the instilled volume, the more rapidly the instilled solution is drained from the precorneal area. Ideally, a high concentration of drug in a minimum drop volume would be desirable. However, there is a practical limit to the concept of minimum dosage volume. Droppers delivering small volumes are difficult to design and to produce. In addition, their practical usefulness could be reduced by the fact that most patients cannot detect the administration of small volumes.
The conjunctival absorption, which occurs via the vessels of the palpebral and scleral conjunctiva, concurs in reducing the drug available for absorption into the eye. Any instilled drug that has not been swept away from the precorneal area by the drainage apparatus is subject to protein binding and to metabolic degradation in the tear film. All of these factors may result in transcorneal absorption of 1% or less of the drug applied topically as a solution. In summary, the rate of loss of drug from the eye can be 500 to 700 times greater than the rate of absorption into the anterior chamber.
Drugs applied topically are potentially available for absorption by the scleral and palpebral conjunctiva (the so-called ‘non-productive’ absorption). Although direct transscleral access to some intraocular tissues cannot be excluded, it is well documented that drugs that penetrate the conjunctiva are rapidly removed from the eye by local circulation and undergo systemic absorption. This may range, for example, from 65% for dipivalylepinephrine to 74% for flurbiprofen and 80% for timolol. [60] These effects are frequently not anticipated, recognised or treated appropriately.
In conclusion, the fluid dynamics in the precorneal area of the eye have a huge effect on ocular drug absorption and disposition. When the normal fluid dynamics are altered by, for example, tonicity, pH or irritant drugs or vehicles, the situation becomes more complex. The formulations of ophthalmic drug products must take into account not only the stability and compatibility of a drug in a given formulation, but also the influence of that formulation on precorneal fluid dynamics. The concepts exposed in this section are summarised in Figure 6, which illustrates the various factors and pathways involved in the ocular disposition of formulations applied topically to the eye.
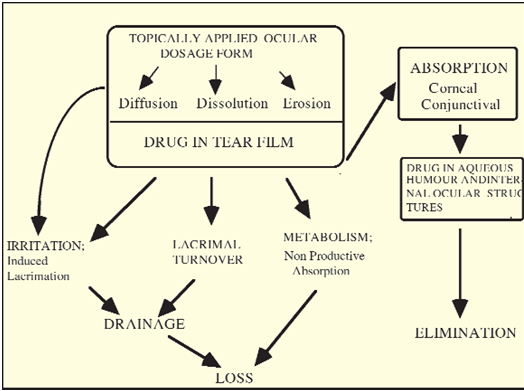
Figure 6: Schematic Illustration of the Ocular Disposition of Topically Applied Formulations
Recent advances and challenges in ocular drug delivery system
Recent advances in topical drug delivery have been made that improve ocular drug contact time and drug delivery, including the development of ointments, gels, liposome formulations and various sustained and controlled-release substrates, such as the Ocusert, collagen shields and hydrogel lenses. The development of newer topical delivery systems using polymeric gels, colloidal systems and cyclodextrins will provide exciting new topical drug therapeutics.[61, 62] The delivery of therapeutic doses of drugs to the tissues in the posterior segment of the eye, however, remains a significant challenge.[63]
Early approaches
A considerable amount of effort has been made in ophthalmic drug delivery since the 1970s. The various approaches attempted in the early stages can be divided into two main categories: bioavailability improvement and controlled release drug delivery. The latter was attempted by various types of inserts and nanoparticles. After initial investigations, some approaches were dropped quickly, whereas others were highly successful and led to marketed products.
Table 1:
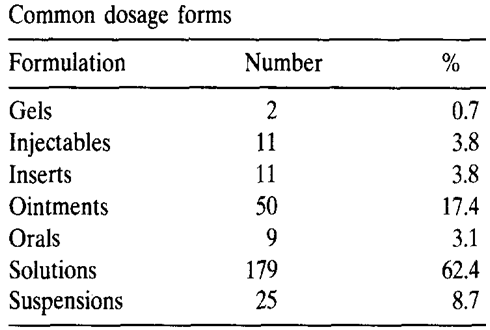
Developments and challenges
Solutions and suspensions
Solutions are the pharmaceutical forms most widely used to administer drugs that must be active on the eye surface or in the eye after passage through the cornea or the conjunctiva. Solutions also have disadvantages: the very short time the solution stays at the eye surface, its poor bioavailability (a major portion, i.e., 75% is lost via nasolacrimal drainage), the instability of the dissolved drug and the necessity of using preservatives. A considerable disadvantage of using eye drops is the rapid elimination of the solution and their poor bioavailability. The retention of a solution in the eye is influenced by viscosity, hydrogen ion concentration, the osmolality and the instilled volume. Extensive work has been done to prolong ocular retention of drugs in the solution state by enhancing the viscosity or altering the pH of the solution. [64-70]
Figure 7: Ophthalmic solution.
Sol to gel systems
The new concept of producing a gel in situ (e.g., in the cul-de-sac of the eye) was suggested for the first time in the early 1980s. It is widely accepted that increasing the viscosity of a drug formulation in the precorneal region leads to an increased bioavailability, due to slower drainage from the cornea. Several concepts for the in situ gelling systems have been investigated. These systems can be triggered by pH, temperature or by ion activation. Middleton and Robinson prepared a sol to gel system with mucoadhesive property to deliver the steroid fluorometholone to the eye. The formulation gave better release of drug over a long period of time in the rabbit’s eye as compared to conventional eye drops. [71]
Figure 8: Ophthalmic gel applied to eye.
Sprays
Although not commonly used, some practitioners use mydriatics or cycloplegics alone or in combination in the form of eye spray. These sprays are used in the eye for dilating the pupil or for cycloplegics examination.
Figure 8: ophthalmic sprays are applied always on closed eyes.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Contact lenses
Contact lenses can absorb water-soluble drugs when soaked in drug solutions. These drug-saturated contact lenses are placed in the eye for releasing the drug for a long period of time. The hydrophilic contact lenses can be used to prolong the ocular residence time of the drugs. In humans, the Bionite lens which was made from hydrophilic polymer (2-hydroxy ethyl methacrylate) has been shown to produce a greater penetration of fluorescein. [72]
Figure 9: Contact lenses.
Artificial tear inserts
A rod shaped pellet of hydroxy propyl cellulose without preservative is commercially available (Lacrisert). This device is designed as a sustained release artificial tear for the treatment of dry eye disorders. It was developed by Merck, Sharp and Dohme in 1981. [73]
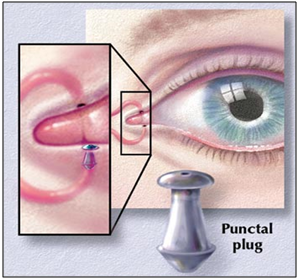
Figure 10: Artificial tear insert.
Filter paper strips
Sodium fluorescein and rose Bengal dyes are commercially available as drug-impregnated filter paper strips. These dyes are used diagnostically to disclose corneal injuries and infections such as herpes simplex and dry eye disorders.
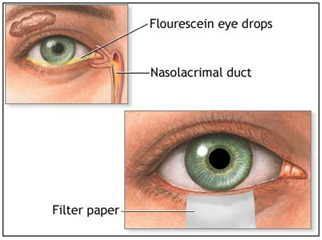
Figure 11: Fluorescein paper strips.
Microemulsion
Due to their intrinsic properties and specific structures, microemulsions are a promising dosage form for the natural defense of the eye. Indeed, because they are prepared by inexpensive processes through auto emulsification or supply of energy and can be easily sterilized, they are stable and have a high capacity of dissolving the drugs. The in vivo results and preliminary studies on healthy volunteers have shown a delayed effect and an increase in the bioavailability of the drug. The proposed mechanism is based on the adsorption of the nanodroplets representing the internal phase of the microemulsions, which constitutes a reservoir of the drug on the cornea and should then limit their drainage. [74-76]

Figure 12: Microemulsion of ocular delivery.
Ocular inserts
Ocular inserts are solid dosage forms and can overcome the disadvantage reported with traditional ophthalmic systems like aqueous solutions, suspensions and ointments. The ocular inserts maintain an effective drug concentration in the target tissues. Limited popularity of ocular inserts has been attributed to psychological factors, such as reluctance of patients to abandon the traditional liquid and semisolid medications and to occasional therapeutic failures (e.g., unnoticed expulsions from the eye, membrane rupture, etc.). A number of ocular inserts were prepared utilizing different techniques to make soluble, erodible, nonerodible and hydrogel inserts. [77–79] The examples of ocular inserts are given in Table 2.
Figure 13: ocular insert.
Table2: Ocular inserts devices [80–88]
|
Name |
Description |
|
Soluble ocular drug Insert
|
Small oval wafer, composed of soluble copolymers consisting of actylamide, N-venyl pyrrolidone and ethyl acetate, soften on insertion |
|
New ophthalmic drug delivery system
|
Medicated solid polyvinyl alcohol flag that is attached to a paper- covered with handle. On application, the flag detaches and gradually dissolves, releasing the drugs
|
|
Collagen shields |
Erodible disc consist of cross-link porcine scleral collagen |
|
Ocusert
|
Flat, flexible elliptical insoluble device consisting of two layers, enclosing a areservior, use commercially to deliver Pilocarpine for 7 days |
|
Minidisc or ocular therapeutic |
system 4-5 mm diameter contoured either hydrophilic or hydrophobic disc |
|
Lacrisert |
Rose-shape device made from Hydroxy propyl cellulose use for the eye syndrome as an alternative to tears
|
|
Bioadhesive ophthalmic eye insets |
Adhesive rods based on a mixture of Hydroxy propyl cellulose, ethyl cellulose, Poly acrylic acid cellulosephthalate |
|
Dry drops |
A preservative free of hydrophilic polymer solution that is freeze dried on the tip of a soft hydrophobic carrier strip, immediately hydrate in tear strip |
|
Gelfoam |
Slabs of Gelfoam impregnated with a mixture of drug and cetyl ester wax in chloroform |
Collagen shield
Collagen is regarded as one of the most useful biomaterials. The excellent biocompatibility and safety due to its biological characteristics such as biodegradability and weak antigenecity made collagen the primary resource in medical applications. Collasomes show promise among drug delivery systems to the human eye. They are first fabricated from porcine scleral tissue, which bears a collagen composition similar to that of the human cornea. The shields are hydrated before they are placed on the eye, having been stored in a dehydrated state. Typically the drug is loaded into the drug solution for a period of time prior to application. Collagen shields are designed to be inserted in a physician’s office; they often produce some discomfort and interfere with vision. Shields are not individually fit for each patient, as are soft contact lenses and therefore, comfort may be problematic and expulsion of the shield may occur. Kaufman et al have developed a new drug delivery system- collasomes. [89] They combined collagen pieces or particles and a viscous vehicle that could be instilled beneath the eyelid, thereby simplifying application and reducing the blurring of vision. Collasomes were well tolerated; and because the collagen particles are suspended in carrier vehicles, they could be instilled safely and effectively by patients in much the same fashion as drops or ointments.
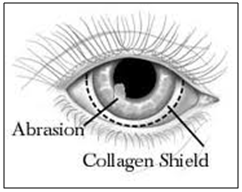
Figure 14: collagen shield loaded with drug inserted in eye.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
Ocular iontophoresis
Iontophoresis is the process in which direct current drives ions into cells or tissues. When iontophoresis is used for drug delivery, the ions of importance are charged molecules of the drug. [90] If the drug molecules carry a positive charge, they are driven into the tissues at the anode; if negatively charged, at the cathode. Ocular iontophoresis offers a drug delivery system that is fast, painless and safe; and in most cases, it results in the delivery of a high concentration of the drug to a specific site. Increased incidence of bacterial keratitis, frequently resulting in corneal scarring, offers a clinical condition that may benefit from drug delivery by iontophoresis. Iontophoretic application of antibiotics may enhance their bactericidal activity and reduce the severity of disease; similar application of anti-inflammatory agents could prevent or reduce vision threatening side effects. [91-92] But the role of iontophoresis in clinical ophthalmology remains to be identified.
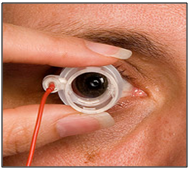
Figure 15: Treatment via ocular ionotophoresis.
Liposomes
Liposomes are phospholipid-lipid vesicles for targeting drugs to the specific sites in the body. They provide controlled and selective drug delivery and improved bioavailability and their potential in ocular drug delivery appears greater for lipophilic than hydrophilic compounds. Liposomes offer the advantage of being completely biodegradable and relatively nontoxic but are less stable than particulate polymeric drug delivery systems. Liposomes were found to be a potential delivery system for administration of a number of drugs to the eye. [93-94]
Niosomes
In order to circumvent the limitations of liposomes, such as chemical instability, oxidative degradation of phospholipids, cost and purity of natural phospholipids, niosomes have been developed as they are chemically stable compared to liposomes and can entrap both hydrophilic and hydrophobic drugs. They are nontoxic and do not require special handling techniques.
Mucoadhesive dosage forms
The successful development of newer mucoadhesive dosage forms for ocular delivery still poses numerable challenges. [95] This approach relies on vehicles containing polymers which will attach, via noncovalent bonds, to conjunctival mucin. Mucoadhesive polymers are usually macromolecular hydrocolloids with numerous hydrophilic functional groups such as carboxyl-, hydroxyl-, amide and sulphate, capable of establishing electrostatic interactions. The bioadhesive dosage form showed more bioavailability of the drug as compared to conventional dosage forms. Thermes et al evaluated the effect of polyacrylic acid as a bioadhesive polymer on the ocular bioavailability of timolol. It was found that polyacrylic acid prolonged the effect of timolol. The pioneering work of Hui and Robinson illustrated the utilization of bioadhesive polymers in the enhancement of ocular bioavailability of progesterone. Subsequently, several natural and synthetic polymers have been screened for their ability to adhere to mucin epithelial surfaces; however, little attention has been paid to their use in ophthalmic drug delivery. [96]
Nanoparticles and microparticles
Particulate polymeric drug delivery systems include micro and nanoparticles. The upper size limit for microparticles for ophthalmic administration is about 5-10 mm. Above this size, a scratching feeling in the eye can result after ocular application. Microspheres and nanoparticles represent promising drug carriers for ophthalmic application.The binding of the drug depends on the physicochemical properties of the drugs, as well as of the nano- or micro-particle polymer. After optimal drug binding to these particles, the drug absorption in the eye is enhanced significantly in comparison to eye drops. Particulates such as nanoparticles, nanocapsules, submicron emulsions, nanosuspensions improved the bioavailability of ocularly applied drugs. [97-99]
Ocular penetration enhancer
Typically classical penetration enhancers have a nonspecific action on biological membranes. They work by reversibly or permanently damaging membranes; therefore, their safety is questionable. Newer penetration enhancers that have been introduced in ocular drug delivery recently with the aim of solving these problems are cyclodextrins, 1-Dodecylazacycloheptan-2-one, Saponin, α-AminoAcid, Pz peptide, etc. Obviously, penetration enhancement has its limit. It is not possible to increase bioavailability indefinitely by use of penetration enhancement alone. Other approaches such as increased residence time and inhibition of metabolizing enzymes should be used in conjunction with penetration enhancement. [100]
Use of hyaluronic acid
The sodium salt of hyaluronic acid (SH) is a high molecular weight biological polymer, made of repeating disaccharide units of glucuronic acid and N-acetyl-b-glucosamine. In the eye, SH is present in the vitreous body and, in lower concentrations, the aqueous humor. SH have several uses in ophthalmic therapy, such as protecting corneal endothelial cells during intraocular surgery, replacing vitreous humor, acting as a tear substitute in the treatment of dry eye and increasing the precorneal residence time of various drugs.
Cyclodextrin
The development of an ophthalmic drug delivery system based on cyclodextrins is relatively recent, occurring in the Patel GM, et al.: Advances and challenges in ocular drug delivery system early 1990s. Cyclodextrins were introduced in ocular drug formulations initially with the aim of increasing the solubility of lipophilic drugs in solution. The authors also observed a decreased toxicity of the drug cyclodextrin complex compared to the usual drug formulation or the prodrug solution (e.g., Pilocarpine Prodrug). Finally, most authors showed increased corneal permeability and increased bioavailability of the drug despite the assumption that the complexes did not penetrate across biological membranes. However, cyclodextrin derivatives possess their own toxicity and these observations vary depending on the studies. Kanai et al (1989) tested different combinations of ~-CD and a lipophilic immunosuppressive agent cyclosporin. They found that the complex of cyclosporin-~-CD resulted in lower corneal toxicity and penetrated into the cornea 5–10 times more than did the drug in a lipophilic vehicle.[101] Cheeks et al confirmed these results in their study on the corneal penetration of cyclosporin.[102] Sasamoto et al, when testing the same cyclosporin-~-cyclodextrin complex to increase drug solubility, recommended topical ~-cyclodextrin-cyclosporin for treating anterior uveitis in rats with the same efficacy as topical fluorometholone solution.[103]
Non-aqueous vehicle
A non-aqueous, comfortable vehicle is desired for topical ophthalmic drug delivery because of drug degradation triggered by water or the premature leakage of the drug from the delivery system in the presence of water. Typically, lipophilic vehicles (that is, mineral oils and vegetable oils) have been used, but they are poorly accepted by patients due to blurred vision and matted eyelids. Reconstitution with water prior to use is unacceptable because of cost and patient compliance issues. Perfluorocarbons or fluorinated silicone liquids have recently been suggested as good non-aqueous vehicles for topical ophthalmic drug delivery. [104] They are chemically and biologically inert and have low surface tension, excellent spreading characteristics and close-to-water refractive indices. Perfluorocarbons have been studied for years as a blood substitute; minimal systemic toxicity is expected via the topical route. [105]
Oxime and methoxime analogs of β-blockers (soft β-blockers for eye targeting)
Several oxime or methoxime analogs of known β-adrenergic blockers are of interest as potential antiglaucoma agents. [106,107] They represent an important class of potential drugs developed using general retro-metabolic drug design principles and can be considered as site-specific enzyme-activated chemical delivery systems (CDSs).[108] The oxime-type CDS approach proposed here provides site-specific or site-enhanced delivery through sequential, multi-step enzymatic and/or chemical transformations [Figure 7]. In the case of eye-targeting CDS, this is achieved through a targetor (T) moiety that is converted into a biologically active function by enzymatic reactions that take place primarily, exclusively or at higher activity at the site of action (i.e., Enz2) as a result of differential distribution of certain enzymes found at the site of action.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
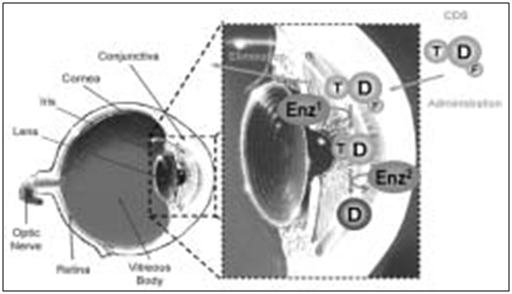
Figure 16: Schematic representation of the processes that provide eye targeting for the oxime-type CDS approach used for eye-targeted delivery of active β-blocker drugs (D)
References
1. Lee VHL, Robinson JR: Topical ocular drug delivery: recent developments and future challenges. Journal of Ocular Pharmacology 1986; 2: 67–108
2. Lang J C. Ocualar drug delivery conventional ocular formulation. Advanced drug delivery review 1995;16:39-43.
3. K Basavaraj, Nanjawade, Manvi FV and Manjappa AS: In situ-forming hydrogels for sustained ophthalmic drug delivery. Journal of Controlled Release 2007; 122: 119–134.
4. Sahoo KS, fahima SAD, kumar K: Nanotechnology in ocular drug delivery, Drug delivery today 2008; 13: 144-151.
5. Weidener J: Mucoadhesive ocular inserts as an improved delivery vehicle for ophthalmic indications. Drug Discovery Today 2003; 8: 906– 907.
6. Lang JC, Roehrs RTE and Jani R: Ophthalmic preparations, Edition 21, vol-1; Lippincott Williams and Wilkins, 2005.
7. Andrews GP, Laverty TP and Jones DS: Mucoadhesive polymeric platforms for controlled drug delivery Review article. European Journal of Pharmaceutics and Biopharmaceutics 2009; 71: 505–518.
8. Alany RG, Rades T, Nicoll J, Tucker IG and Davies NM: W/O microemulsions for ocular delivery: Evaluation of ocular irritation and precorneal retention. Journal of Controlled Release 2006; 111: 145-152.
9. Binstock EE and Domb AJ: Iontophoresis: A non-invasive ocular drug delivery. Journal of Controlled Release 2006; 110: 479–489.
10. Gazayerly, E.L., Omaima. N. and Hikal. A H., Int. J. Pharm. 1997; (158); 121.
11. Chien YW: Ocular drug delivery and delivery systems, special edition, 269-296.
12. Greaves JL and Wilson CG: Treatment of diseases of the eye with mucoadhesive delivery systems. Advance Drug Delivery Review 1993; 11: 349– 383.
13. Robinson JC: Ocular anatomy and physiology relevant to ocular drug delivery, Ophthalmic Drug Delivery Systems, New York, A.K. Mitra Edition, 1993, 29–57.
14. Huang AJW, Tseng SCG and Kenyon KR: Paracellular permeability of corneal and conjunctival epithelia. Investigation Ophthalmology of Visual Sciense 1989; 30: 684– 689.
15. Haeringen NJV: Clinical biochemistry of tears. Survival Ophthalmology 1981; 5 : 84– 96.
16. Nagyova B and Tiffany JM: Components responsible for the surface tension of human tears. Current Eye Research 1999; 19 : 4 – 11.
17. Ahmed L, Gokhale RD, Shah MV and Paton TF: Phytochemical determinants of drug difussion across the conjunctiva, sclera and cornea. Journal of Pharmaceutical Science 1987: 76 : 583-587.
18. A. Urtti, L. Salminen, Minimizing systemic absorption of topically administered ophthalmic drugs, Surv. Ophthalmol. 37 (1993) 435–457.
19. A. Urtti, L. Salminen, O. Miinalainen, Systemic absorption of ocular pilocarpine is modified by polymer matrices, Int. J. Pharm. 20(1985) 147–161.
20. A. Urtti, H. Rouhiainen, T. Kaila, V. Saano, Controlled ocular timolol delivery: systemic absorption and intraocular pressure effects in humans, Pharm. Res. 11 (1994) 1278–1282.
21. A. Urtti, J.D. Pipkin, G.S. Rork, T. Sendo, U. Finne, A.J. Repta, Controlled drug delivery devices for experimental ocular studies with timolol. 2. Ocular and systemic absorption in rabbits, Int. J. Pharm. 61 (1990) 241–249.
22. D.M. Maurice, S. Mishima, Ocular pharmacokinetics, in: M.L. Sears (Ed.), Handbook of experimental pharmacology, vol. 69, Springer Verlag, Berlin-Heidelberg, 1984, pp. 16–119.
23. M. Hornof, E. Toropainen, A. Urtti, Cell culture models of the ocular barriers, Eur. J. Pharm. Biopharm. 60 (2005) 207–225.
24. H.S. Huang, R.D. Schoenwald, J.L. Lach, Corneal penetration behavior of beta-blockers, J. Pharm. Sci. 72 (1983) 1272–1279.
25. M.R. Prausnitz, J.S. Noonan, Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye, J. Pharm. Sci. 87 (1998) 1479–1488.
26. K.M. Hämäläinen, K. Kontturi, L. Murtomäki, S. Auriola, A. Urtti, Estimation of pore size and porosity of biomembranes from permeability measurements of polyethylene glycols using an effusion-like approach, J. Control. Release 49 (1997) 97–104.
27. D.H. Geroski, H.F. Edelhauser, Transscleral drug delivery for posterior segment disease, Adv. Drug Deliv. Rev. 52 (2001) 37–48.
28. Dartt DA, Hodges RR, Zoukhri D. Tear and Their Secretion. In: Fischbarg J (ed). The biology of the eye. New York: Academic Press; 2006: 21-82.
29. Macha S, Mitra AK. Overview of ocular drug delivery. In: Mitra AK (ed). Ophthalmic drug delivery systems. 2nd ed. New York: Marcel Dekker; 2003: 1-12.
30. Mitra AK, Anand BS, Duvvuri S. Drug delivery to the eye. In: Fischbarg J (ed). The biology of the eye. New York: Academic Press; 2006: 307-351.
31. Ahmed I. The noncorneal route in ocular drug delivery. In: Mitra AK (ed). Ophthalmic drug delivery systems. 2nd ed. New York: Marcel Dekker; 2003: 335-363.
32. Huang HS, Schoenwald RD, Lach JL. Corneal penetration behavior of beta-blocking agents II: Assessment of barrier contributions. J Pharm Sci 1983;72:1272-1279.
33. Sunkara G, Kompella UB. Membrane transport processes in the eye. In: Mitra AK (ed). Ophthalmic drug delivery systems. New York: Marcel Dekker; 2003: 13-58.
34.Schoenwald RD, Huang HS. Corneal penetration behavior of beta-blocking agents I: physiochemical factors. J Pharm Sci 1983;72:1266-1272.
35. Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci 1989;30:684-689.
36. Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 1997;38:627-634.
37. Janoria KG, Gunda S, Boddu SH, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv 2007;4:371-388.
38. Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther 2003;3:45-56.
39. Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 1985;26:584-587.
40. J.W. Sieg, J.R. Robinson, Mechanistic studies on transcorneal penetration of pilocarpine, J. Pharm. Sci. 65 (1976) 1816–1822.
41. A.L. Gomes dos Santos, A. Bochot, A. Doyle, N. Tsapis, J. Siepmann, F. Siepmann, J. Schmaler, M. Besnard, F. Behar-Cohen, E. Fattal, Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery, J. Control. Release 112 (2006) 369–381.
42. Z.F. Bashshur, A. Bazarbachi, A. Schakal, Z.A. Haddad, C.P. El Haibi, B.N. Noureddin, Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration, Am. J. Ophthalmol. 142 (2006) 1–9.
43. B. Zhou, B. Wang, Pegaptanib for the treatment of age-related macular degeneration, Exp. Eye Res. 83 (2006) 615–619.
44. L. Pitkänen, V.P. Ranta, H. Moilanen, A. Urtti, Permeability of retinal pigment epithelium: effect of permeant molecular weight and lipophilicity, Investig. Ophthalmol. Vis. Sci. 46 (2005) 641–646.
45. J. Ambati, E.S. Gragoudas, J.W. Miller, T.T. You, K. Miyamoto, F.C. Delori, A.P. Adamis, Transscleral delivery of bioactive protein to the choroid and retina, Investig. Ophthalmol. Vis. Sci. 41 (2000) 1186–1191.
46. L. Pitkänen, M. Ruponen, J. Nieminen, A. Urtti, Vitreous is a barrier in non-viral gene transfer by cationic lipids and polymers, Pharm. Res. 20 (2003) 576–583.
47. J. Park, P.M. Bungay, R.J. Lutz, J.J. Augsburger, R.W. Millard, A.S. Roy, R.K. Banerjee, Evaluation of coupled convective– diffusive transport of drugs administered by intravitreal injection and controlled release implant, J. Control. Release 105 (2005) 279–295.
48. Jain N.K, Menqui S.A and Deshpande S.G. “ Controlled and Novel Drug Delivery”, CBS publishers; New Delhi;1ST Edition(2005); 82
49. J C Lang, “Ocular drug delivery: conventional ocular formulations”, Adv. Drug Delivery Revs, 16, 1995, pp. 39–43.
50. S P Eriksen (1980), “Physiological and formulation constraints on ocular drug bioavailability”, Ophthalmic Drug Delivery Systems, (Ed. J R Robinson), American Pharmaceutical Association, Washington, pp. 55–70.
51. S Mishima, “Clinical pharmacokinetics of the eye”, Invest. Ophthalmol. Vis. Sci., 21, 1981, pp. 504–541.
52. J W Shell, “Pharmacokinetics of topically applied ophthalmic drugs”, Surv. Ophthalmol., 26, 1982, pp. 207–218.
53. D M Maurice and S Mishima (1984), “Ocular Pharmacokinetics”, Pharmacology of the Eye, (Ed. M L Sears), Springer Verlag, New York, pp. 19–116.
54. J W Shell, “Ophthalmic drug delivery systems”, Surv. Ophthalmol., 29, 1984, pp. 117–128.
55. N L Burstein and J A Anderson, “Corneal penetration and ocular bioavailability of drugs”, J. Ocular Pharmacol., 1, 1985, pp. 309–326.
56. R D Schoenwald (1993), “Ocular Pharmacokinetics/Pharmacodynamycs”, Ophthalmic Drug Delivery Systems, (Ed. A K Mitra), M Dekker, Inc., New York, pp. 83–110.
57. K Järvinen, T Järvinen and A Urtti, “Ocular absorption following topical delivery”, Adv. Drug Delivery Revs, 16, 1995, pp. 3–19.
58. M F Saettone (1995), “Effect of different vehicles on ocular kinetics/distribution”, Ocular Toxicology, (Eds. I Weisse, O Hockwin, K Green and R C Tripathi), Plenum Press, New York and London, pp. 109–120.
59. J Himmelstein, I Guvenir and T F Patton, “Preliminary pharmacokinetic model of pilocarpine uptake and distribution in the eye”, J. Pharm. Sci., 67, 1978, pp. 603–606.
60. V H L Lee (1993), “Precorneal Corneal and Postcorneal factors”, Ophthalmic Drug Delivery systems, (Ed. A K Mitra), M Dekker, Inc., New York, pp. 59–82.
61. Le Bourlais C, Acnar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems - recent advances. Prog Retinal Eye Res 1998;17:33-58.
62. Ding S. Recent developments in ophthalmic drug delivery. Pharmaceutical 1998;1:328-35.
63. Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci 2000;41:961-4.
64. Mueller WH, Deardroff DL. Ophthalmic vehicles: The effect of methyl cellulose on the penetration of Homatropine hydro bromide through the cornea. J Am Pharma Assoc 1956;45:334-41.
65. Krishna N, Brown F. Polyvinyl alcohol as an ophthalmic vehicle. Am J Ophthalmol 1964;57:99-106.
66. Swanson AA, Jeter DJ, Tucker P. Ophthalmic vehicles II. Comparison of ointment and polyvinyl alcohol 1.4%. Ophthalmologica 1970;160:265-70.
67. Wattman SR, Patrowicz TC. Effects of hydroxypropyl methyl cellulose and polyvinyl alcohol on intraocular penetration of topical fluorescein in man. Invest Ophthalmol 1970;9:966-70.
68. Schoenwald RD, Smolen VF. Drug-absorption analysis from pharmacological data II: Transcorneal biphasic availability of tropicamide. J Pharma Sci 1971;60:1039-45.
69. Benedetto DA, Shah DO, Kaufman HE. The instilled fluid dynamics and surface chemistry of polymers in the precorneal tear film. Invest Ophthalmol 1975;14:887-902.
70. Trueblood JH, Rossmando RM, Carlton WH, Wilson LA. Corneal contact times of ophthalmic vehicles. Arch Ophthalmol 1975;93:127-30.
71. Middleton DL, Robinson JR. Design and evaluation of an ocular bioadhesive delivery system. STP Pharma Sci 1991;1:200-6.
72. Vadnere M, Amidon G, Lindenbaum S, Haslam JL. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Int J Pharma 1984;22:207-18.
73. La Motte J, Grossman E, Hersch J. The efficacy of cellulosic ophthalmic inserts for treatment of dry eye. J Am Optom Assoc 1985;56:298-302.
74. Vandamme TF. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog Retin Eye Res 2002;21:15-34.
75. Ding S Tien W, Olejnik O. US Patent 1995;5:474-979.
76. Ding S, Olejnik O. Pharma Res 1997;14:S41.
77. Lerman S, Davis P, Jackson WB. Prolonged release hydrocortisone therapy. Can J Ophthalmol 1973;8:114-8.
78. Hosaka S, Ozawa H, Tanzawa H. Controlled release of drug from hydrogel matrices. J Appl Polym Sci 1979;23:2089.
79. Ozawa H, Hosaka S, Kunitomo T, Tanzawa H. Ocular inserts for controlled release of antibiotics. Biomaterials 1984;4:170-4.
80. Vasantha R, Sehgal PK, Rao P. Collagen ophthalmic inserts for Pilocarpine drug delivery system. Int J Pharma 1988;47:95-102.
81. Bawa R. Ocular inserts. In: Mitra AK, editor. Ophthalmic drug delivery systems. Marcel Dekker Inc: New York, US; 1993. p. 223-60.
82. Lewrenson GJ, Edgae DF, Gudgeon AC, Burns JM, Geraint M, Nas BA. Comparision of the efficiency and duration of action of topically applied Poxymetacaine using a novel ophthalmic drug delivery system versus eye drops in healthy young voluenteers. Br J Ophalmol 1993;77:713-5.
82. O’Brien TP, Sawusch MR, Dick DJ, Green KL, Smart JD. Use of corneal collagen shields versus soft contact lances to enhance penetration of topical Tobramycin. J Cataract Refract Surg 1998;14:505-7.
83. Quigley HA, Pollacc IP, Herbin TS, Pilocarpin ocuserts. Long term clinical trials and selected pharmacodynamics. Arch Ophalmol 1975;93:771-5.
84. Hetoni P, Di Cilo G, Grandi M, Morroli M, Saettone MF, Darougar S. Silicon rubber/Hydrogel composite ophthalmic inserts preparation and preliminary in-vitro/in-vivo evaluation. Eur J Pharma Biopharmacetics 1995;46:125-32.
85. Guetler F, Kaltsatosh V, Bioserame B, Gurny R, Long acting soluble Bioadhesive Ophalmic Drug Inserts (BODI) containing Gentamycin for veterinary use: Optimization and clinical investigation. Hiunal Controlled Release 1995;33:231-6.
86. Diestelhorst M, Grunthal S, Suverkrup R. Dry Drops: A new preservative free Drug delivery system. Graefes Arch Clin Exp Opthalmol 1999;237:94.
87. Sinamora P, Nadkarni SR, Lee YC, Yalkowsky SH. Controlled delivery of pilocarpine. 2. Invitro evaluation of Gelfoam device. IJPS 1998;170:209-14.
88. Kaufman HE, Steinemann TL, Lehman E, Thompson HW, Varnell ED, Jacob-Labarre T, et al. J Ocul Pharma 1994;10:17-27.
89. Hill JM, O’Callaghan RJ, Hobden JA. Ocular Iontophoresis. In: Mitra AK, editor. Ophthalmic drug delivery systems. Marcel Dekker Inc: New York; 1993. p. 331-54.
90. Rootman DS, Jantzen JA, Gonzalez JR, Fischer MJ, Beuerman R, Hill JM. Pharmacokinetics and safety of transcorneal iontophoresis of tobramycin in the rabbit. Invest Ophthalmol Vis Sci 1988;29:1397-401.
91. Callegan MC, Hobden JA, O’Callaghan RJ, Hill JM. Ocular drug delivery: A comparison of transcorneal iontophoresis to corneal collagen shields. Int J Pharma 1995;123:173-9.
92. Nagarsenkar MS, Vaishali Y, Londhe, Nadkarni GD. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharma 1999;190:63- 71.
93. Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharma 2000;198:29-38.
94. Mitra AK. Opthalmic drug delivery system. Marcel Dekker, 2nd ed. In: Mucoadhesive polymers in ophalmic. Drug Delivery 2003;130:409-33.
95. Hui HW, Robinson JR. Ocular Delivery of Progesterone using a bioadhesive polymer. Int J Pharma 1985;26:203.
96. Swanson AA, Jeter DJ, Tucker P. Ophthalmic vehicles II. Comparison of ointment and polyvinyl alcohol 1.4%. Ophthalmologica 1970;160:265-70.
97. Wattman SR, Patrowicz TC. Effects of hydroxypropyl methyl cellulose and polyvinyl alcohol on intraocular penetration of topical fluorescein in man. Invest Ophthalmol 1970;9:966-70.
98. Schoenwald RD, Smolen VF. Drug-absorption analysis from pharmacological data II: Transcorneal biphasic availability of tropicamide. J Pharma Sci 1971;60:1039-45.
99. Mitra AK. Opthalmic drug delivery system. Marcel Dekker. 2nd ed. In: Ocular Penetration Enhancers 2003;130:281-307.
100. Kanai A, Alba RM, Takano T, Kobayashi C, Nakajima A, Kurihara K, et al. The effect on the cornea of alpha cyclodextrin vehicle for cyclosporin eye-drops. Transplant Proc 1989;21:3150-2.
101 Cheeks L, Kaswan RL, Green K. Influence of vehicle and anterior chamber protein concentration on cyclosporin penetration through the isolated rabbit cornea. Curr Eye Res 1992;11:641-9.
102. Sasamoto Y, Hirose S, Ohno S, Ono K, Matsuda H. Topical application of cyclosporin ophthalmic solution containing alpha-cyclodextrin in experimental uveitis. Ophthalmologica 1991;18:25.
103. Meadows DL. US Patent 1992;5:173-298.
104. Riess JG, Krafft MP Artif cells blood substitutes and immobilization. Biotechnol 1997,43-52
105. Bodor N, El-Koussi A, Kano M, Nakamuro T. Improved delivery through biological membranes. Design, synthesis and pharmacological activity of a novel chemical delivery system for âadrenergic blocking agents. J Med Chem 1988;31:100-6.
106. Simay A, Prokai L, Bodor N. Oxidation of aryloxy-amino alcohols with activated dimethylsulfoxide: A novel C-N oxidation facilitated by neighboring group effect. Tetrahedron 1989;45:4091-102.
107. Bodor N. Drug targeting and retrometabolic drug design approaches. Adv Drug Deliv Rev 1994;14:157-66.
108. Peyman GA, Ganiban GJ. Delivery systems for intraocular. Routes. Adv Drug Deliv Rev 1997;16:107-23.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org











.png)

