About Author:
Mohit Dangwal
DIT Faculty of Pharmacy
Dehradun, Uttarakhand
dangwal_mohit@yahoo.in
Abstract
Transdermal drug delivery system (TDDS) is topically administered dosage form in the form of patches which deliver drugs for systemic effects at a predetermined and controlled rate. This review focuses towards the basic facts about the transdermal drug delivery system including the methods of their preparation and some of the recent advancements that have in achieved in this field.
REFERENCE ID: PHARMATUTOR-ART-2015
INTRODUCTION
Transdermal drug delivery system (TDDS) is the dosage forms which deliver a therapeutically effective amount of drug across a patient’s skin [1]. Transdermal drug delivery offers controlled release of the drug into the patient, it enables a steady blood-level profile resulting in reduced systemic side effects and sometimes, painless and offer multi-day dosing. Since the first transdermal patch was approved in 1981 to prevent the nausea and vomiting associated with motion sickness, the FDA has approved, throughout the past 22 years, more than 35 transdermal patch products, spanning 13 molecules[2].
Advantages [3]
- This approach to drug delivery offers many advantages over traditional methods
- Transdermal drug delivery enables the avoidance of gastrointestinal absorption, with its associated pitfalls of enzymatic and pH associated deactivation
- This method also allows for reduced pharmacological dosaging due to the shortened metabolization pathway of the transdermal route versus the gastrointestinal pathway.
- The simplified medication regimen leads to improved patient compliance and reduced inter & intra – patient variability.
- Self administration is possible with these systems.
- The drug input can be terminated at any point of time by removing transdermal patch.
Disadvantages [4]
- The drug that requires high blood levels cannot be administered and may even cause irritation or sensitization of the skin
- Only relatively potent drugs are suitable candidates for TDDS because of the natural limits of drug entry imposed by the skin’s impermeability.
- High cost of the product is also a major drawback for the wide acceptance of this product
- Contact dermatitis is another drawback reported in TDDS
- Barrier function of skin varies from site to site during application in same person
HUMAN SKIN[5]
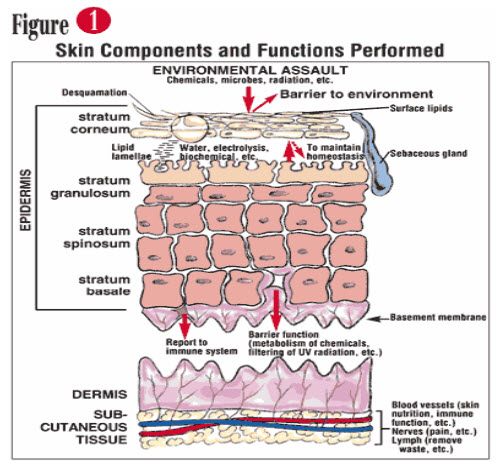
Fig: SKIN COMPONENTS AND FUNCTIONS PERFORMED ROUTES FOR DRUG PENETRATION THROUGH SKIN –
There are macro and micro routes for drug penetration through the skin
1. Macro route -
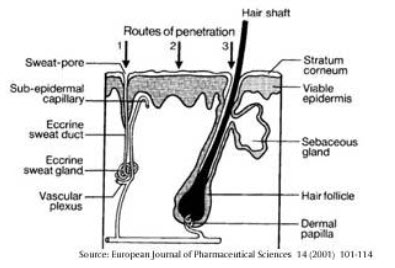
FIGURE : DRUG PERMEATION THROUGH THE SKIN
2.Microroute –
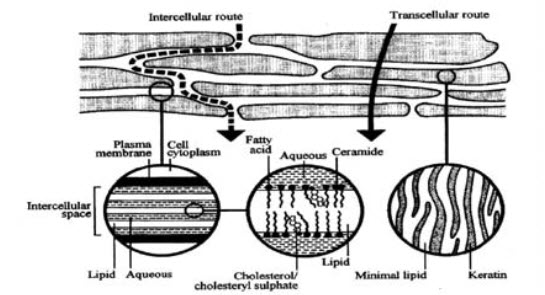
FIGURE : MECHANISAM OF SKIN PERMEATION
Components of TDDS
- Polymer matrix / Drug reservoir
- Drug
- Permeation enhancers
- Pressure sensitive adhesive (PSA)
- Backing laminates
- Release liner
- Other excipients
1:Polymer matrix / Drug reservoir:[6]
These control the release rate of the drug from the device and are hence considered as the backbone of the system.These are generally prepared by dispersing the drug in liquid or solid state synthetic polymeric base.
Possible useful polymers for transdermal devices are: [7,8,9,10,11]
(a).Natural Polymers: e.g., cellulose derivatives, Zein, Gelatin, Shellac, Waxes, Proteins, Gums and their derivatives, Natural rubber, Starch etc.
(b).Synthetic Elastomers: e.g. polybutadiene, hydrin rubber, polyisobutylene, silicon rubber, nitrile, acrylonitrile, neoprene, butylrubber etc.
(c).Synthetic Polymers: e.g. polyvinyl alcohol, polyvinylchloride, polyethylene, polypropylene, polyacrylate, polyamide, polyurea, polyvinylpyrrolidone, polymethylmethacrylate etc.
2:Drug:[12,13]
Ideal Properties of a Transdermal Drug Delivery System are as follows
|
S.no |
Properties |
Comments |
|
1
|
Shelf life |
Up to 2 yrs |
|
2
|
Particle size |
<40cm2 |
|
3
|
Dosing frequency |
Once in a day or once in week |
|
4
|
Aesthetic appeal |
Clear or white colour |
|
5
|
Packaging |
Easy removal of release liner & min. no. of steps required to apply |
|
6
|
Skin irritation |
Easy removal of release liner & min. no. of steps required to apply |
|
7
|
Release |
Consistent pharmacokinetic & pharmacodynamic profile over time. |
Permeation enhancers[14,15,16,17]
The chemical compounds that increase permeability of stratum corneum so as to attain higher therapeutic levels of the drug candidate are called permeation enhancers. These interact with structural components of stratum corneum i.e.,proteins or lipids and alter the protein and lipid packaging of stratum corneum and modifying chemically the barrier functions which increases permeability
Commonly used penetration enhancers are:
a. Solvents:
These agents enhances the penetration by swallowing the polar pathway and/or by fluidizisation of lipids
Examples include water alcohols – methanol and ethanol; alkyl methyl sulfoxides – dimethyl sulfoxide, alkyl homologs of methyl sulfoxide dimethyl acetamide and dimethyl formamide; pyrrolid- ones- 2 pyrrolidone, N-methyl, 2-purrolidone; etc
(b)Surfactants:
These agents are beneficial when the drug is hydrophilic in nature.The surfactant alter the permeation by polar head group and hydrocarbon chain length
Anionic Surfactants: e.g. Decodecylmethyl sulphoxide, Dioctyl sulphosuccinate, Sodium lauryl sulphate etc.
Nonionic Surfactants: e.g. Pluronic F127, Pluronic F68, etc.
Bile Salts: e.g.Sodium ms taurocholate, Sodium tauroglycocholate, Sodium deoxycholate
Binary system:These system open up the heterogeneous multilaminate pathway as well as the continuous pathways.e.g. Propylene glycol-oleic acid and 1, 4-butane diol-linoleic acid.
(C) Miscellaneous agents: These include urea, a hydrating and keratolytic agent; N, N- dimethylm- toluamide;anticholinergic agent: calcium thioglycolate.
4. Pressure sensitive adhesives(PSA)[18,19]
A PSA is a material which maintains an intimate contact between transdermal system and the skin surface. It should adhere with not more than applied finger pressure, be aggressively and permanently tachy, and exert a strong holding force. Additionally, it should be removable from the smooth surface without leaving a residue. Silicones based adhesives and polyacrylates, polyisobutylene are mostly used in TDDS
5. Backing Laminate:[20]
The consideration of chemical resistance of the material is most important while designing a backing layer. Excipient compatibility should also be considered because the prolonged contact between the backing layer and the excipients may cause the additives to leach out of the backing layer or may lead to diffusion of excipients, drug or penetration enhancer through the layer. Backing which exhibits lowest modulus or high flexibility, good oxygen transmission and a high moisture vapor transmission rate are considered for backing. Examples of some backing materials are vinyl, polyethylene and polyester films is considered an excellent backing.
6. Release Liner:[20]
It is a protective liner that is removed and discharged immediately before the application of the patch to skin during application of patch. It is therefore regarded as a part of the primary packaging material rather than a part of dosage form for delivering the drug. Typically, release liner is composed of a base layer which may be non-occlusive (e.g. paper fabric) or occlusive (e.g. polyethylene, polyvinylchloride) and a release coating layer made up of silicon or Teflon. Other materials used for TDDS release liner include polyester foil and metallized laminates
7. Other excipients:[19,20]
Solvents such as chloroform, methanol, acetone, isopropanol and dichloromethane are used to prepare drug reservoir. In addition plasticizers such as dibutylpthalate, triethylcitrate, polyethylene glycol and propylene glycol are added to provide plasticity to the transdermal patch
Classification of transdermal drug delivery system[21,22,23]
1. Matrix System : Drug in Adhesive System (Adhesive Diffusion Controlled TDDS)
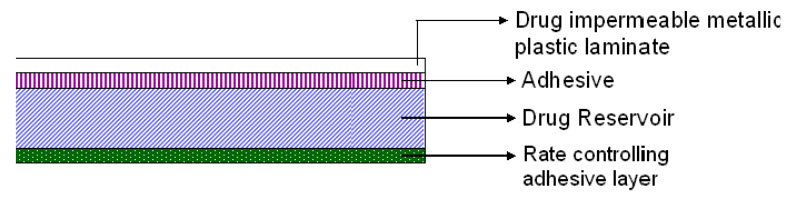
FIGURE 1: DRUG IN ADHESIVE TYPE OF PATCH
The drug reservoir is formed by dispersing the drug in an adhesive polymer and then spreading the medicated polymer adhesive by solvent casting or by melting the adhesive (in case of hot melt adhesives) onto an impervious backing layer. The drug reservoir layer is then covered by a non-medicated rate controlling adhesive polymer of constant thickness to produce an adhesive diffusion controlling drug delivery system. Deponit® (Nitroglycerine) for once a day medication of angina pectoris.
2. Matrix System : Matrix Dispersion System (Matrix Diffusion Controlled System)
The drug is dispersed homogeneously in a hydrophilic or lipophilic polymer matrix. This drug containing polymer disk then is fixed onto an occlusive base plate in a compartment fabricated from a drug-impermeable backing layer. Instead of applying the adhesive on the face of the drug reservoir, it is spread along the circumference to form a strip of adhesive rim. Nitro Dur® (Nitroglycerine) used for once a day medication of angina pectoris.
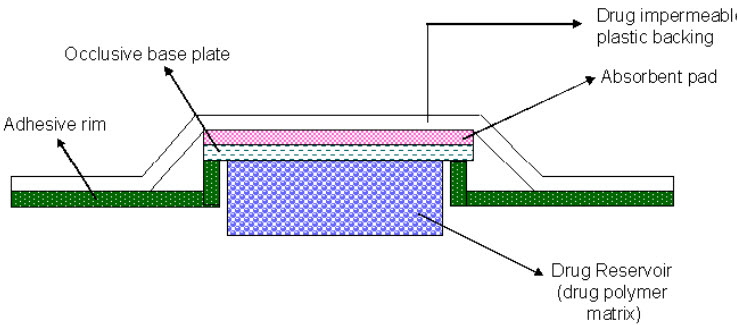
FIGURE 2: MATRIX DISPERSION SYSTEM
3. Reservoir System (Membrane Moderated TDDS):
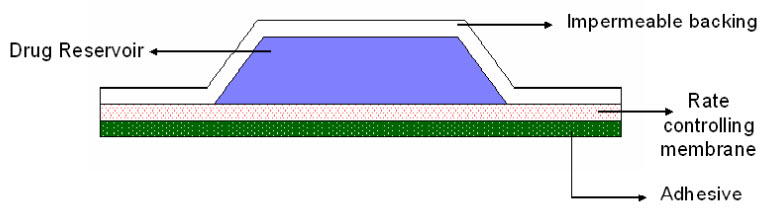
FIGURE 3: RESERVOIR TRANSDERMAL PATCH
In this system, the drug reservoir is embedded between an impervious backing layer and a rate controlling membrane. The drug releases only through the rate controlling membrane, which can be microporous or non-porous. In the drug reservoir compartment, the drug can be in the form of a solution, suspension, or gel or dispersed in solid polymer matrix. On the outer surface of the polymeric membrane a thin layer of drug-compatible, hypoallergenic adhesive polymer can be applied. The rate of drug release from this type of transdermal drug delivery system can be tailored by varying the polymer composition, permeability coefficient and thickness of the rate controlling membrane
TransdermScop® (Scopolamine) for 3 days protection of motion sickness and TransdermNitro® (Nitroglycerine) for once a day medication of angina pectoris.
4.Microreservoir System
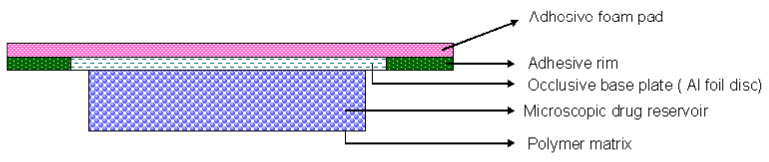
FIGURE 4: MICRORESERVOIR SYSTEM
This drug delivery system is a combination of reservoir and matrix-dispersion systems. The drug reservoir is formed by first suspending the drug in an aqueous solution of water-soluble polymer and then dispersing the solution homogeneously in a lipophilic polymer to form thousands of unleachable, microscopic spheres of drug reservoirs. The thermodynamically unstable dispersion is stabilized quickly by immediately cross-linking the polymer in situ. A transdermal system therapeutic system thus formed as a medicated disc positioned at the centre and surrounded by an adhesive rim. Nitro-dur® System (Nitroglycerin) for once a day treatment of angina pectoris.
METHODS FOR PREPARATION of TDDS:
a. Asymmetric TPX membrane method[25]
A prototype patch can be fabricated for this a heat sealable polyester film (type 1009, 3m) with a concave of 1cm diameter will be used as the backing membrane. Drug sample is dispensed into the concave membrane, covered by a TPX {poly (4-methyl-1-pentene)} asymmetric membrane, and sealed by an adhesive.
b. Circular Teflon mould method[26]
Solutions containing polymers in various ratios are used in an organic solvent. Calculated amount of drug is dissolved in half the quantity of same organic solvent. Enhancers in different concentrations are dissolved in the other half of the organic solvent and then added. Di-N-butylphthalate is added as a plasticizer into drug polymer solution. The total contents are to be stirred for 12 hrs and then poured into a circular Teflon mould. The moulds are to be placed on a leveled surface and covered with inverted funnel to control solvent vaporization in a laminar flow hood model with an air speed of 0.5 m/s. The solvent is allowed to evaporate for 24 hrs. The dried films are to be stored for another 24 hrs at 25±0.5°C in a desiccators containing silica gel before evaluation to eliminate aging effects. The type films are to be evaluated within one week of their preparation.
c. Mercury substrate method[27]
In this method drug is dissolved in polymer solution along with plasticizer. The above solution is to be stirred for 10-15 minutes to produce a homogenous dispersion and poured in to a leveled mercury surface, covered with inverted funnel to control solvent evaporation.
d. By using IPM membranes” method[28]
In this method drug is dispersed in a mixture of water and propylene glycol containing carbomer 940 polymer and stirred for 12 hrs inmagnetic stirrer. The dispersion is to be neutralized and made viscous by the addition of triethanolamine. Buffer pH 7.4 can be used inorder to obtain solution gel, if the drug solubility in aqueous solution is very poor. The formed gel will be incorporated in the IPMmembrane.
e. By using EVAC membranes” method[29]
In order to prepare the target transdermal therapeutic system, 1% carbopol reservoir gel, polyethelene (PE), ethylene vinyl acetate copolymer (EVAC) membranes can be used as rate control membranes. If the drug is not soluble in water, propylene glycol is used for the preparation of gel. Drug is dissolved in propylene glycol; carbopol resin will be added to the above solution and neutralized by using 5% w/w sodium hydroxide solution. The drug (in gel form) is placed on a sheet of backing layer covering the specified area. A rate controlling membrane will be placed over the gel and the edges will be sealed by heat to obtain a leak proof device.
f. Aluminium backed adhesive film method[30]
Transdermal drug delivery system may produce unstable matrices if the loading dose is greater than 10 mg. Aluminium backed adhesive film method is a suitable one. For preparation of same, chloroform is choice of solvent, because most of the drugs as well as adhesive are soluble in chloroform. The drug is dissolved in chloroform and adhesive material will be added to the drug solution and dissolved. A custam made aluminium former is lined with aluminium foil and the ends blanked off with tightly fitting cork blocks
g. Preparation of TDDS by using Proliposomes[31]
The proliposomes are prepared by carrier method using film deposition technique. From the earlier reference drug and lecithin in the ratio of 0.1:2.0 can be used as an optimized one. The proliposomes are prepared by taking 5mg of mannitol powder in a 100 ml round bottom flask which is kept at 60-70°c temperature and the flask is rotated at 80 90rpm and dried the mannitol at vacuum for 30 minutes. After drying, the temperature of the water bath is adjusted to 20-30°C. Drug and lecithin are dissolved in a suitable organic solvent mixture, a 0.5ml aliquot of the organic solution is introduced into the round bottomed flask at 37°C, after complete drying second aliquots (0.5ml) of the solution is to be added. After the last loading, the flask containing proliposomes are connected in a lyophilizer and subsequently drug loaded mannitol powders proliposomes) are placed in a desiccator over night and then sieved through 100 mesh. The collected powder is transferred into a glass bottle and stored at the freeze temperature until characterization.
h. By using free film method[32]
Free film of cellulose acetate is prepared by casting on mercury surface. A polymer solution 2% w/w is to be prepared by using chloroform. Plasticizers are to be incorporated at a concentration of 40% w/w of polymer weight. Five ml of polymer solution was poured in a glass ring which is placed over the mercury surface in a glass petri dish. The rate of evaporation of the solvent is controlled by placing an inverted funnel over the Petri dish. The film formation is noted by observing the mercury surface after complete evaporation of the solvent. The dry film will be separated out and stored between the sheets of wax paper in a desiccators until use. Free films of different thickness can be prepared by changing the volume of the polymer solution.
EVALUATION OF TRANSDERMAL PATCHES[33,34,35,36]
Evaluation studies for the transdermal dosage forms can be classified into following types:-
(1) Physicochemical evaluation
(2) In vitro evaluation
(3) In vivo evaluation
(1) Physicochemical Evaluation:
(a) Thickness:
The thickness of transdermal film is determined by, dial gauge, screw gauge or micrometer, traveling microscope at different points of the film.
(b) Uniformity of weight:
Weight variation is studied through individually weighing 10 randomly selected patches and calculating the average weight. The individual weight should not deviate significantly from the average weight.
(c) Flatness:
For flatness determination, one narrow piece is cut from the centre and two from each side of patches. The length of each strip is measured and variation in length is measured by determining percent constriction. Zero percent constriction is equivalent to 100 percent flatness.
(d) Tensile Strength:
To determine tensile strength of the transdermal patches. The polymeric films in the transdermal patch are sandwiched individually by corked linear iron plates. One end of the films is kept fixed with the help of an iron screen and other end is connected to a freely movable thread over a pulley. The weights are added gradually to the pan close with the hanging end of the thread. A pointer on the thread is used to measure the elongation of the film. The weight just sufficient to break the film is noted. The tensile strength can be calculated using the following equation.
Tensile strength= F/a. b (1+L/l)
F is the force required to break, a is width of film, b is thickness of film, L is length of film, l is elongation of film at break point.
(e) Moisture content:
The prepared films are weighed individually and put in a desiccators containing calcium chloride at room temperature for 24 h. The films are weighed again after a specified interval until they show a constant weight. The percent moisture content is calculated using following formula.
%Moisture content =Initial weight –Final weight X 100
Final weight
(f) Drug content determination:
Accurately weighed portion of film (about 100 mg) is dissolved in 100 ml of suitable solvent in which drug is soluble and then the solution is shaken continuously for 24 h in shaker incubator. Then the whole solution is sonicated. After sonication and subsequent filtration, drug in solution is estimated spectrophotometrically by appropriate dilution.
(g) Content uniformity test:
10 patches are selected and content is determined for individual patches. If 9 out of 10 patches have content between 85% to 115% of the specified value and one has content not less than 75% to 125% of the specified value, then transdermal patches pass the test of content uniformity. But if 3 patches have content in the range of 75% to 125%, then additional 20 patches are tested for drug content. If these 20 patches have range from 85% to 115%, then the transdermal patches pass the test.
(h) Moisture Uptake:
Weighed films are put in a desiccator at room temperature for 24 h. These are then taken out and exposed to 84% relative humidity using saturated solution of Potassium chloride in a desiccator until a constant weight is achieved. % moisture uptake is calculated as given below.
% moisture uptake = Final weight – Initial weight X 100
Initial weight
(i) Folding Endurance:
Evaluation of folding endurance involves determining the folding capacity of the films subjected to frequent extreme conditions of folding. Folding endurance is determined by repeatedly folding the film at the same place until it break. The number of times the films could be folded at the same place without breaking is folding endurance value.
(j) Microscopic studies:
Microscopic evaluation is used to determine the sharing of drug and polymer in the film can be studied using scanning electron microscope. For this study, the sections of each sample are cut and then mounted onto stubs using double sided adhesive tape. The sections are then coated with gold palladium alloy using fine coat ion sputter to render them electrically conductive. Then the sections are examined under scanning electron microscope.
(k) Adhesive studies:
The adhesive studies are used to determine the adhesive properties of TDDS. The therapeutic performance of TDDS can be affected by the quality of contact between the patch and the skin.
The adhesion of a TDDS to the skin is obtained by using PSAs, which are defined as adhesives capable of bonding to surfaces with the application of light pressure. The adhesive properties of a TDDS can be characterized by considering the following factors.
Peel Adhesion properties: It is the force necessary to remove adhesive coating from test substrate. It is tested by measuring the force required to pull a single coated tape, applied to substrate at 180° angle. The test is passed if there is no residue on the substrate.
Tack properties: It is the ability of the polymer to adhere to substrate with little contact pressure. Tack is dependent on molecular weight and composition of polymer as well as on the use of tackifying resins in polymer.
Thumb tack test: The force necessary to remove thumb from adhesive is a measure of tack.
Rolling ball test: This test involves measurement of the distance that stainless steel ball travels along an upward facing adhesive. The less tacky the adhesive, the further the ball will travel.
Quick stick (Peel tack) test: The peel force required breaking the bond between an adhesive and substrate is measured by pulling the tape away from the substrate at 90º at the speed of 12 inch/min.
Probe tack test: Force required to pull a probe away from an adhesive at a fixed rate is recorded as tack.
(2) In vitro evaluation:
(a) In vitro release studies:
In vitro release studies performed outside the body. This is used to determine the drug release mechanism and kinetics are two characteristics of the dosage forms which play an important role in describing the drug dissolution profile from a controlled release dosage forms. The dissolution data explain the release mechanism of the drug.
There are different methods available for determination of drug release rate of TDDS.
The paddle over disc: In this method is identical to the USP paddle dissolution apparatus is used, except that the transdermal system is attached to a disc or cell resting at the bottom of the vessel which contains medium at 32 ±5°C. (USP apparatus 5)
The cylinder modified USP BasketThis method is like to the USP basket type dissolution apparatus, except that the system is attached to the surface of a hollow cylinder immersed in medium at 32 ±5°C. (USPapparatus 6)
The reciprocating disc:
In this method patches attached to holders are oscillated in small volumes of medium, allowing the apparatus to be useful for systems delivering low concentration of drug. In addition paddle over extraction cell method may be used. (USP apparatus 7)
Diffusion cells: E.g. Franz Diffusion Cell and its modification Keshary- Chien Cell. These areused for determine the release rate of drug from tansdermal dosage forms.
(b) In vitro permeation studies: It is used to determine the permeation of the drug from the transdermaldosage form. The amount of drug available for absorption to the systemic pool is greatlydependent on drug released from the polymeric transdermal films. The drug reached at skinsurface is then passed to the dermal microcirculation by penetration through cells of epidermis,between the cells of epidermis through skin appendages. Usually permeation studies areperformed by placing the fabricated transdermal patch with rat skin or synthetic membrane inbetween receptor and donor compartment in a vertical diffusion cell such as franz diffusion cellor keshary-chien diffusion cell.
(3) In vivo evaluation: In vivo evaluation of the transdermal dosage form performed in site the body. Invivo evaluations are the true description of the drug performance. The variables which cannot betaken during in vitro studies can be fully explored during in vivo studies.
In vivo evaluation of TDDS can be carried out using:
(a) Animal models
(b) Human volunteers
(a) Animal models: The most common animal species used for evaluating transdermal drug delivery system are mouse, hairless rhesus monkey, hairless rat, hairless dog, , rabbit, guinea pig etc.Various experiments conducted lead us to a conclusion that hairless animals are preferred over hairy animals in both in vitro and in vivo experiments. Rhesus monkey is one of the most reliable models for in vivo evaluation of transdermal drug delivery in man.
(b) Human volunteers: Human models are used for gathering of pharmacokinetic and pharmacodynamic performance data of the transdermal dosage forms Clinical trials have been conducted to evaluate the efficacy, risk involved, side effects, patient compliance etc. Phase I clinical trials are conducted to find out mainly safety in volunteers and phase II clinical trials find out short term safety and mainly effectiveness in patients. Phase III trials indicate the safety and effectiveness in large number of patient population and Phase IV trials at post marketing surveillance are done for marketed patches to detect adverse drug reactions. Though human studies require significant resources but they are the best to assess the performance of the drug.
Stability studies: The stability studies are conducted to find out the effect of temperature and relative humidity on the drug content in different formulations. The transdermal formulations are subjected to stability studies as per ICH guidelines.
Skin irritation studies:
White albino rats, mice or white rabbits are used to study any hypersensitivity reaction on the skin. Mutalik and Udupa (2005) carried out skin irritation test using mice.
Transdermal Drug Delivery: Past, Present, Future:[37]
TABLE : CHARACTERISTICS OF SEVERAL DRUGS DELIVERED TRANSDERMALLY
|
Active Ingredient |
Mol. Wt. (Daltons) |
Trade Name(s) |
Daily Dose |
Frequency of Application |
Type of System |
|
Clonidine |
230 |
Catapres-TTS® |
0.1–0.3 mg |
Weekly |
Reservoir |
|
Estradiol |
272 |
Esclim® |
0.025–0.1 mg |
Weekly |
Drug-in-adhesive |
|
Vivelle® |
|||||
|
Vivelle-Dot® |
|||||
|
Climara® |
|||||
|
Ethinyl Estradiol |
296 |
Ortho-Evra® |
0.15 mg |
Weekly |
Drug-in-adhesive |
|
Norelgestromin |
328 |
0.02 |
|||
|
Fentanyl |
337 |
Duragesic |
0.6 mg |
Once every three days |
Reservoir |
|
Transdermal System® |
|||||
|
Lidocaine |
234 |
Lidoderm® |
Not stated |
Daily |
Drug-in-adhesive |
|
Nicotine |
162 |
Nicoderm CQ® |
7–21 mg |
Daily |
* |
|
Nicotrol® |
|||||
|
Nitroglycerine |
227 |
Nitro-Dur® |
1.4–11.2 mg |
Daily |
Drug-in-adhesive |
|
Nitrodisc® |
Reservoir |
||||
|
Scopolamine |
303 |
Transderm-Scop® |
0.33 mg |
Once every three days |
Reservoir |
|
Testosterone |
288 |
Androderm® |
2.5–5 mg |
Daily |
Reservior |
Advance Development in TDDS:
Drug in adhesive technology has become the preferred system for passive transdermal delivery; two areas of formulation research are focused on adhesives and excipients. Adhesive research focuses on customizing the adhesive to improve skin adhesion over the wear period, improve drug stability and solubility, reduce lag time, and increase the rate of delivery. Because a one-size-fits-all adhesive does not exist that can accommodate all drug and formulation chemistries, customizing the adhesive chemistry allows the transdermal formulator to optimize the performance of the transdermal patch
A rich area of research over the past 10 to 15 years has been focused on developing transdermal technologies that utilize mechanical energy to increase the drug flux across the skin by either altering the skin barrier (primarily the stratum corneum) or increasing the energy of the drug molecules. These so-called “active” transdermal technologies include iontophoresis (which uses low voltage electrical current to drive charged drugs through the skin), electroporation (which uses short electrical pulses of high voltage to create transient aqueous pores in the skin), sonophoresis (which uses low frequency ultrasonic energy to disrupt the stratum corneum), and thermal energy (which uses heat to make the skin more permeable and to increase the energy of drug molecules). Even magnetic energy, coined magnetophoresis, has been investigated as a means to increase drug flux across the skin
CONCLUSION
In the development of transdermal patches of TDDS a lot of process has been used. Many researchers are interested in the field of TDDS. Due to large benefit of the TDDS, many new researchers are going on in the development of transdermal dosage forms of newer drug via this system in the present day.
REFERENCES
1. Loyd V. Allen Jr, Nicholas G. Popovich, Howard C. Ansel. Pharmaceutical dosage forms and drug delivery systems, 8th Edition., Wolter Kluwer Publishers,New Delhi, 2005 pp. 298-299
2. Kumar R, Philip A. Modified Transdermal Technologies: Breaking the Barriers of Drug Permeation via the Skin. Trop J Pharm Res. 2007, 6(1):633-644.
3. Brahmankar.D.M, Jaiswal.S.B, Biopharmaceutics and pharmacokinetics A Teatise. Vallabh Prakashan, Delhi1995, 335-371.
4. Ansel.H.C, Loyd.A.V, Popovich.N.G, Pharmaceutical dosage forms and drug delivery systems, Seventh edition, Lippincott Williams and Willkins publication.
5. Skin available at; en.wikipedia.or/wiki/skin
6. Bromberg L. Cross linked polyethylene glycol networks as reservoirs for protein delivery, Apply Poly Sci 1996, 59, 459-466.
7. Verma PRP, Iyer SS. Transdermal delivery of propranolol using mixed grades of eudragit: Design and in vitro and in vivo evaluation, Drug Dev Ind Pharm 2000, 26, 471-476
8. Ubaidulla U, Reddy MV, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: Effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics, AAPS PharmSciTech 2007, 8(1), Article 2.
9. Gannu R, Vamshi Vishnu Y, Kishan V, Madhusudan Rao Y. Development of nitrendipine transdermal patches: In vitro and ex vivo characterization, Current Drug Delivery 2007, 4, 69-76.
10. Chung SJ. Future drug delivery research in South Korea, J Controlled Release 1999, 62, 73 79.
11. Chung SJ. Future drug delivery research in South Korea, J Controlled Release 1999, 62, 73 79.
12. Izumoto T, Aioi A, Uenoyana S, Kariyama K, Azuma M. Relationship between the transference of drug from a transdermal patch andphysicochemical properties, Chem Pharm Bull (Tokyo) 1992, 40, 456-458.
13. Gordon RA, Peterson TA. Four myths about transdermal drug delivery, Drug Delivery Technology 2003, 3, 1-7.
14. Williams AC, Barry BW. Penetration enhancers, Advanced drug delivery reviews 2004, 56, 603-618.
15. Shin SC, Shin EY, Cho CY. Enhancing effects of fatty acids on piroxicam permeation through rat skins, Drug Dev Ind Pharm. 2000, 26, 563-566.
16. Pocius AV. Adhesives. In: Howe- Grants M, Ed. Kirk-Othmer Encyclopedia of Chemical Technology. New York, Wiley-Interscience. 1991; 445-466.
17. Walters KA. Transdermal drug delivery systems In: Swarbick K, Boylan JC, eds. Encyclopedia of pharmaceutical technology. New York, Marcel Dekker Inc. 1997; 253-293.
18.Tan HS, Pfister WR. Pressure sensitive adhesives for transdermal drug delivery, Pharm Sci Technol Today 1999, 2, 60-69.
19. Pfister WR, Hsieh DS. Permeation enhancers compatible with transdermal drug delivery systems. Part I: Selection and formulationconsiderations, Med Device Technol 1990, 1, 48-55.
20. Godbey KJ. Improving patient comfort with nonocclusive transdermal backings, America











.png)

