 About Authors:
About Authors:
Darveshwar Jagdeep D*, Mule Madhav S1, Birajdar Shivprasad M2
*NRI Institute of Pharmaceutical Science, Bhopal, India
1School of Pharmacy, Swami Ramanand Teerth Marathwada University
Vishnupuri, Nanded-431606, Maharashtra, India,
2Department of Quality Assurance, Maharashtra College of Pharmacy,
Nilanga-413521, Maharashtra, India
*jagdeepdd@hotmail.com, birajdar100@gmail.com
Abstract:
Polymers have played indispensable roles in the preparation of pharmaceutical products. Their applications range widely from material packaging to fabrication of the most sophisticated drug delivery devices. This review includes various polymers used in pharmaceutics based on their applications. The review focuses on the use of pharmaceutical polymer for controlled drug delivery applications. Examples of pharmaceutical polymers and the principles of controlled drug delivery are outlined and applications of polymers for controlled drug delivery are described. The field of controlled drug delivery is vast therefore this review aims to provide an overview of the applications of pharmaceutical polymers. General pharmaceutical applications of polymers in Dental Medicine, Ophthalmic Drug Delivery, Gene Delivery, Preparation of micro spheres etc. are also discussed briefly.
Reference Id: PHARMATUTOR-ART-1509
INTRODUCTION
Life on Earth is linked to the presence of water, and water is our main constituent. However, all kinds of living species are not simply ‘bags of water’ but are highly organized. This specialized organization depends on other compounds, macromolecules, i.e. polymers that are able to retain and structure water, as natural hydro gels. Many polymers have a natural origin (mineral, vegetal or animal). Some of these have been used for centuries. In the vegetal kingdom, cellulose is the most abundant macromolecule. Cellulose is a polysaccharide composed of repeating units of cellobiose, i.e. it is a dimer of glucose. In the animal kingdom, chitin, a polymer of N-acetyl glucosamine, is widely distributed as the main constituent of the shell of arthropods. Proteins and nucleic acids are well known as supports of life, and these natural polymers retain and structure water. Use, including in the biomedical and pharmaceutical fields. An even wider diversity of properties and uses has been obtained with the development of synthetic polymers, which can be prepared by different methods and processes of polymerization of one or more monomers. In the domain of health, a very interesting property of purely synthetic polymers is their absence of immunogenicity, unlike many polymers of natural origin.
Definition
“Polymers are long chain organic molecules assembled from many smaller molecules called as monomers.”
In pharmaceutical preparations also they have several applications in mfg of bottles, syringes, vials, catheters, and also in drug formulations
Classification
A. Based on origin:
a) Natural Polymers: e.g. Proteins – Collagen, Keratin, Albumin Carbohydrates – starch, cellulose, glycogen.
b) Synthetic Polymers: e.g. polyesters, polyanhydrides, polyamides.
B. Based on Bio-stability:
a) Bio-degradable Polymers:
E.g. polyesters, proteins, carbohydrates, etc
b) Non – biodegradable Polymers:
E.g. ethyl cellulose, HPMC, acrylic polymer
Characteristics of Ideal Polymer
- Should be inert and compatible with the environment.
- Should be non-toxic.
- Should be easily administered.
- Should be easy and inexpensive to fabricate.
- Should have good mechanical strength
APPLICATIONS IN CONVENTIONAL DOSAGE FORMS
In Solid Dosage Forms,
1. Tablets
2. Capsules
3. Film Coatings of Solid Dosage Forms
4. Disperse Systems
5. Gels
6. Transdermal Drug Delivery Systems (Patches)
Tablets
In tablet the polymer are used as a Binder and Disintegrants. Binders which bind the powder particle in a damp mass various polymer are used are Ethyl cellulose, HPMC, Starch, Gelatin, polyvenylpyrrolidine. Alginic acid, Glucose, Sucrose. Disintegrates like Starch, cellulose, Alginates, polyvenylpyrrolidine, sodium CMC which decrease the time of dissolution and gives fast action of drug.
Capsules
The various polymer are used in the capsule as the plasticizer on which the flexibility and strength of the Gelatin are depend on it .The release rate of the Capsule are controlled by using the various type of polymer.
Naturalcoating agents
Natural polymer like Shellac and zein, although still used from time to time, are hardly able to meet present-day requirements. Organic solvents should be reserved for special applications only and chlorinated hydrocarbons such as methylene chloride and chloroform are avoided altogether, since they impose a heavy load on the environment. Low-molecular-weight types of methylcellulose and hydroxypropyl methylcellulose can also be processed as aqueous solutions. Ethyl cellulose and cellulose acetate phthalate are available as aqueous dispersions, so-called pseudolatexes. An overview of the most widely used cellulosic’s is presented, the structure and properties of acrylic polymers.
The solubility properties of EUDRAGIT® acrylic polymers are adjusted to the conditions of the digestive tract. They satisfy particularly stringent requirements in terms of purity. Further quality characteristics are the high stability to environmental influences during storage and absolute skin friendliness, i.e. indifference to bodily tissue and fluids. The amount of acrylic polymer consumed with the active ingredient is very small, only a few milligrams in the case of coated tablets and approximately 150 mg per day with specific sustained-release preparations. The average polymer quantity taken up by an adult is thus about 2 mg per kg of body weight.
Disperse Systems
The biphasic system are like emulsion, suspension use various polymer for disperse one phase into another phase i.e. water phase disperse in oil phase or vice versa the polymer like poly vinyl pyrolidine, ethyl cellulose etc. Dispersed Systems consist of particulate matter known as the dispersed phase, distributed throughout the dispersion medium with the help of dispersing agent polymer mentioned above. In the oil in water in oil type emulsion the dispersion of drug content is very difficult but it is easily produced by using polymer as a dispersing agent.
Film Coatings of Solid Dosage Forms
Chitosan's film forming abilities lend itself well as a coating agent for conventional solid dosage forms such as tablets. Furthermore its gel- and matrix-forming abilities make it useful for solid dosage forms, such as granules, micro particles, etc. Sakkinen and coworkers studied microcrystalline chitosan as gel-forming excipients for matrix-type drug granules. Crystallinity, molecular weight, and degree of deacetylation were seen to be factors that affected the release rates from the chitosan-based granules. Combination of positively charged chitosan with negatively charged biomolecules, such as gelatin, alginic acid, and hyalouronic acid, has been tested to yield novel matrices with unique characteristics for controlled release of drugs
Taste masked by spray drying:
Chitosan and drug are dissolved in suitable solvent. Sonication done by ultracentrifuge, after stirring 24 hrs with magnetic stirrer, after completely loading drug to polymer, complex dried by spray drying and evaluated for taste masking, Threshold concentration of bitterness. Complexes characterization done with the help of XRPD, FT-IR, DSC and SEM. If Complexation was achieve, % of drug content was determine and equivalent weight of complexes taken and formulate it. Dissolution of the chitosan – drug complexes tablet give sustain released effect.
Transdermal Drug Delivery Systems (Patches)
In the formulation of Transdermal Patches various polymer are used. The baking material also prepared from the polymer for supporting of drug in drug reservoir.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
APPLICATIONS IN CONTROLLED DRUG DELIVERY
1. Reservoir Systems
2. Ocusert System
3. Matrix Systems
4. Swelling Controlled Release Systems
5. Biodegradable Systems
6. Osmotically controlled Drug Delivery
7. Introduction: Principles of Controlled Drug Delivery
8. The Progestasert System
9. Reservoir Designed Transdermal Patches
10. Matrix Systems
11. Stimulus Responsive Drug Release
12. Ultrasound Responsive Drug Release
13. Temperature Responsive Drug Release
14. pH Responsive Drug Release
15. Electric Current Responsive Drug Release
16. Polymer-Drug Conjugates
POLYMERS IN BIOMEDICAL APPLICATIONS
Water-Soluble Synthetic Polymers
· Poly (acrylic acid) Cosmetic, pharmaceuticals, immobilization of cationic drugs, base for Carbopol polymers
· Poly (ethylene oxide) Coagulant, flocculent, very high molecular-weight up to a few millions, swelling agent
· Poly (ethylene glycol) MW <10,000; liquid (MW <1000) and wax (MW >1000), plasticizer, base for suppositories
· Poly (vinyl pyrrolidone) Used to make betadine (iodine complex of PVP) with less toxicity than iodine, plasma replacement, tablet granulation
· Poly (vinyl alcohol) Water-soluble packaging, tablet binder, tablet coating Polyacrylamide Gel electrophoresis to separate proteins based on their molecular weights, coagulant, absorbent.
· Poly (isopropyl acrylamide) and poly (cyclopropyl methacrylamide)
· Thermogelling acrylamide derivatives, its balance of hydrogen bonding, and hydrophobic association changes with temperature
Cellulose-Based Polymers
· Ethyl cellulose Insoluble but dispersible in water, aqueous coating system for sustained release applications
· Carboxymethyl cellulose Super disintegrant, emulsion stabilizer
· Hydroxyethyl and hydroxypropyl celluloses
· Soluble in water and in alcohol, tablet coating
· Hydroxypropyl methyl cellulose Binder for tablet matrix and tablet coating, gelatin alternative as capsule material
· Cellulose acetate phthalate Enteric coating
Hydrocolloids
· Alginic acid Oral and topical pharmaceutical products; thickening and suspending agent in a variety of pastes, creams, and gels, as well as a stabilizing agent for oil-in-water emulsions; binder and disintegrant
· Carrageenan :- Modified release, viscosifier
· Chitosan :- Cosmetics and controlled drug delivery applications, mucoadhesive dosage forms, rapid release dosage forms
· Hyaluronic acid Reduction of scar tissue, cosmetics
· Pectinic acid Drug delivery
Water-Insoluble Biodegradable Polymers
· Lactide-co-glycolide polymers Microparticle–nanoparticle for protein delivery.
Starch-Based Polymers
· Starch Glidant, a diluent in tablets and capsules, a disintegrant in tablets and capsules, a tablet binder Sodium starch glycolate Super disintegrant for tablets and capsules in oral delivery
Plastics and Rubbers
· Polyurethane Transdermal patch backing (soft, comfortable, moderate moisture transmission), blood pump, artificial heart, and vascular grafts, foam in biomedical and industrial products Silicones Pacifier, therapeutic devices, implants, medical grade adhesive for transdermal delivery
· Polycarbonate Case for biomedical and pharmaceutical products
· Polychloroprene Septum for injection, plungers for syringes, and valve components
· Polyisobutylene Pressure sensitive adhesives for transdermal delivery
· Polycyanoacrylate Biodegradable tissue adhesives in surgery, a drug carrier in nano- and microparticles
· Poly (vinyl acetate) Binder for chewing gum
· Polystyrene Petri dishes and containers for cell culture
· Polypropylene Tight packaging, heat shrinkable films, containers
· Poly (vinyl chloride) Blood bag and tubing.
· Polyethylene Transdermal patch backing for drug in adhesive design, wrap, packaging, containers
· Poly (methyl methacrylate) Hard contact Lenses Poly (hydroxyethyl methacrylate) Soft contact lenses
APPLICATION IN PARENTERAL
In Parenteral the various polymer like Methacrylic acid act as an Interferon inductor which induce to the interferon in cancer like disease. Methacrylic acid alkyl amide is act as plasma expander which increase the plasma level in body when admixture of drug with polymer present in body. Some Vaccines are transpired by using polymer because which disintegrate in GIT tract, example Methyl methacrylate.
In the disease diabetes the insulin are administered by using different polymer reservoir which form bond with insulin and release at target site.
NUTRITIONAL APPLICATION
Cholesterol lowering effect
Chitosanbind cholesterol, fat and initiate clotting of RBC. Fibers with a range of abilities to perturb cholesterol homeostasis were used to investigate how the serum cholesterol-lowering effects of insoluble dietary fibers are related to parameters of intestinal cholesterol absorption and hepatic cholesterol homeostasis in mice. Cholestyramine, chitosan and cellulose were used as examples of fibres with high, intermediate and low bile acid-binding capacities, respectively. The serum cholesterol levels in a control group of mice fed a high fat/high cholesterol (HFHC) diet for 3 weeks increased about 2-fold to 4·3mM and inclusion of any of these fibres at 7·5% of the diet prevented this increase from occurring. In addition, the amount of cholesterol accumulated in hepatic stores due to the HFHC diet was reduced by treatment with these fibres. The three kinds of fibres showed similar hypocholesterolaemic activity; however, cholesterol depletion of liver tissue was greatest with cholestyramine. The mechanisms underlying the cholesterol-lowering effect of cholestyramine were,
1) Decreased cholesterol (food) intake,
2) Decreased cholesterol absorption efficiency, and
3) Increased faecal bile acid and cholesterol excretion.
The latter effects can be attributed to the high bile acid-binding capacity of cholestyramine. Incontrast, incorporation of chitosan or cellulose in the diet reduced cholesterol (food) intake, but did not affect either intestinal cholesterol absorption or faecal sterol output. The present study provides strong evidence that above all satiation and satiety effects underlie the cholesterol-lowering.
Weight loss effect
Chitosan use to replace calories in blood and food, which increase the body weight.
CUTANEOUS APPLICATION
Carpool, Meth acrylic acid, Methyl Meth acrylic acid, Methyl Meth acrylic acid are used in the Ointment, Gel, Transversalsystem, WoundSpray, MucosalOintment. The polymer like In terms of structure and electronics, melanins are "rigid-backbone" conductive polymerscomposed of polyacetylene, polypyrrole, and polyaniline "Blacks" and their mixed copolymers. The simplest melanin is polyacetylene, and some fungal melanins are pure polyacetylene. synthetic melanin (commonly referred to as BSM, or "black synthetic matter") is made up of 3-6 oligomeric units linked together — the so-called "protomolecule" — there is no evidence that naturally occurring biopolymer (BCM, for "black cell matter") mimics this structure. Evidence exists in support of a highly cross-linked heteropolymerbound covalentlyto matrix scaffolding melanoproteins. It has been proposed that the ability of melanin to act as an antioxidantis directly proportional to its degree of polymerization or molecular weight.
APPLICATION IN COSMETICS
Cosmetic compositions are disclosed for the treatment of hair or skin, characterized by a content of new quaternary chitosan derivatives of the formula. The chitosan derivatives have a good substantively, particularly to hair keratin, and prove to have hair strengthening and hair conditioning characteristics. e.g.; Hair setting lotion, Oxidation Hair-coloring Composition, Hair toning Composition, Skin Cream, Hair-treatment Composition, Gel-form.
APPLICATION IN MANUFACTURING
1.Bottle
2.Vials
3.Syringes
4.Baking material
GENERAL PHARMACEUTICAL APPLICATIONS
Increase stability of drug:
Chitosan is use to increase the stability of the drug in which the drug is complexes with chitosan and make slurry and kneading for 45 minutes until dough mass. This dough mass is pass-through sieve no.16 and make a granules is completely stable at different condition.
Orthopaedic patients:
Chitosan is a biopolymer that exhibits osteo conductive, enhanced wound healing and antimicrobial properties which make it attractive for use as a bioactive Coating to improve Osseo integration of orthopedic and craniofacial implant devices. It has been proven to be useful in promoting tissue growth in tissue repair and accelerating wound-healing and bone regeneration.
Enhanced bone formation by transforming growth factor
Chitosan composite microgranules were fabricated as bone substitutes for the purpose of obtaining high bone-forming efficacy. The microgranules have the flexibility to fill various types of defect sites with closer packing. The interconnected pores formed spaces between the microgranules, which allowed new bone in growth and vascularization. In addition, the transforming growth factor-beta 1 (TGF-pl) was incorporated into the microgranules in order to improve bone-healing efficacy. The chitosan microgranules were fabricated by dropping a mixed solution into a NaOH/ethanol solution. TGF-pl was loaded into the chitosan microgranules by soaking the microgranules in a TGF-pl solution.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Dental Medicine
Chitin / chitosan have been recognized to accelerate wound healing to attain an aesthetically valid skin surface, and to prevent excess scar formation. In dental medicine, chitin / chitosan is also applied as a dressing for oral mucous wound and a tampon following radical treatment of maxillary sinusitis. Furthermore, it is being investigated as an absorbing membrane for periodoental surgery. Chitin / chitosan has a variety of biological activities and advertised as a healthy food that is effective for improvement and/or care of various disorders, arthritis, cancer, diabetes, hepatitis, etc. In Japan , it is renowned since a three-year old Russian boy whose skin was burnt 90 % in total area
For oral drug delivery: Preliminary study on film dosage form
The potential of chitosan films containing diazepam as an oral drug delivery was investigated inrabbits. The results indicated that a film composed of a 1:0.5 drug-chitosan mixture might be an effective dosage form that is equivalent to the commercial tablet dosage forms. The ability of chitosan to form films may permit its use in the formulation of film dosage forms, as an alternative to pharmaceutical tablets. The pH sensitivity, coupled with the reactivity of the primary amine groups, make chitosan a unique polymer for oral drug delivery applications.
Permeation Enhancer:
It has been reported that chitosan, due to its cationic nature is capable of opening tight junctions in a cell membrane. This property has led to a number of studies to investigate the use of chitosan as a permeation enhancer for hydrophilic drugs that may otherwise have poor oral bioavailability, such as peptides. Because the absorption enhancement is caused by interactions between the cell membrane and positive charges on the polymer, the phenomenon is pH and concentration dependant. Furthermore increasing the charge density on the polymer would lead to higher permeability.
Mucoadhesive Excipient:
Bioadhesivity is often used as an approach to enhance the residence time of a drug in the GI tract, thereby increasing the oral bioavailability. A comparison between chitosan and other commonly used polymeric excipients indicates that the cationic polymer has higher bioadhesivity compared to other natural polymers, such as cellulose, Xantham gum, and starch.
Ophthalmic Drug Delivery:
Chitosan exhibits favorable biological behavior, such as bioadhesion, permeability-enhancing properties, and interesting physico-chemical characteristics, which make it a unique material for the design of ocular drug delivery vehicles. Due to their elastic properties, chitosan hydro gels offer better acceptability, with respect to solid or semisolid formulation, for ophthalmic delivery, such as suspensions or ointments, ophthalmic chitosan gels improve adhesion to the mucin, which coats the conjunctiva and the corneal surface of the eye, and increase precorneal drug residence times, showing down drug elimination by the lachrymal flow. In addition, its penetration enhancement has more targeted effect and allows lower doses of the drugs. In contrast, chitosan based colloidal system were found to work as transmucosal drug carriers, either facilitating the transport of drugs to the inner eye (chitosan-coated colloidal system containing indomethacin) or their accumulation into the corneal/conjunctival epithelia (chitosan nanoparticulate containing cyclosporine). The micro particulate drug- carrier (micro spheres) seems a promising means of topical administration of acyclovir to the eye. The duration of efficacy of the ofloxacin was increased by using high MW (1930 kd) chitosan.
Gene Delivery:
The course of many hereditary diseases could be reversed by gene delivery. In addition, many acquired diseases such as multigenetic disorders and those diseases caused by viral genes could be treated by genetic therapy. Gene delivery systems include viral vectors, cationic liposomes, polycation complexes, and microencapsulated systems. Viral vectors are advantageous for gene delivery because they are highly efficient and have a wide range of cell targets. However, when used in vivo they cause immune responses and oncogenic effects. To overcome the limitations of viral vectors, non-viral delivery systems are considered for gene therapy. Non-viral delivery system has advantages such as ease of preparation, cell/tissue targeting, low immune response, unrestricted plasmid size, and large-scale reproducible production. Chitosan has been used as a carrier of DNA for gene delivery applications. Also, Chitosan could be a useful oral gene carrier because of its adhesive and transport properties in the GI tract. MacLaughlin et al. Showed that plasmid DNA containing cytomegalo virus promoter sequence and a luciferase reporter gene could be delivered in vivo by Chitosan and depolymerized Chitosan oligomers to express a luciferase gene in the intestinal tract.
Preparation of micro spheres:
A novel cellulose acetate/chitosan multimicrospheres (CA/CM) was prepared by the method of w/o/w emulsion. The concentration of cellulose acetate (CA) and the ratio of CA/chitosan (CS) had influence on the CACM size, and appearance. Ranitidine hydrochloride loading and releasing efficiency in vitro were investigated. The optimal condition for preparation of the microspheres was CA concentration at 2% and the ratio of CA/CS at 3:1. The microspheres size was 200–350 μm. The appearance of microspheres was spherical, porous, and non aggregated. The highest loading efficiency was 21%. The ranitidine release from the CACM was 40% during 48 hr in buffers.
Polymethacrylates for pharmaceutical purposes
Neutral poly(meth)acrylates are pharmacologically inactive. Good compatibility with the skin and mucous membranes prompted their use for wound sprays and ointment bases. Crosslinked copolymers based on methacrylic acid serve as ion exchangers for adsorption of active ingredients in the manufacture of sustained-release formulations in the form of tablets and suspensions. For sustained release, active ingredients can also be embedded in water-insoluble polymers, e.g. by compression to tablets together with polymer powders or by extrusion at the softening temperatures of the polymers between 120 and 200 °C. Probably the most important role of poly (meth) acrylates in pharmaceutical manufacture is that of special excipients for coating oral dosage forms and for ensuring controlled release of the active ingredient. Coating of tablets, sugar-coated products, capsules, granules, pellets, crystals and other drug-loaded cores serves to ensure their physical and chemical stability, to enhance patient compliance and to further improve their therapeutic efficacy. Acknowledging the fact that the efficiency of a pharmaceutical dosage form depends not only on the active ingredients it contains but also, and critically so, on the formulation and processing technique, scientists and engineers alike have devoted increasing attention to these parameters in recent years.
Pharmaceutical applications of Chitosan polymer in various dosage forms:
Due to its good biocompatibility and low toxicity properties in both conventional exciepient applications as well as in novel application, chitosan has received considerable attention as a pharmaceutical exceipient in recent decades.
Medical
The polymer Polyvenylpyrolidin was used as a blood plasma expander for trauma victims after the first half of the 20th century. It is used as a binder in many pharmaceutical tablets; it simply passes through the body when taken orally. However, autopsies have found that crosspovidone does contribute to pulmonary vascular injury in substance abusers who have injected pharmaceutical tablets intended for oral consumption. The long-term effects of crosspovidone within the lung are unknown. PVP added to iodine forms a complex called povidone-iodine that possesses disinfectant properties. This complex is used in various products like solutions, ointment, pessaries, liquid soaps and surgical scrubs. It is known for instance under the trade name Betadine. It is used in pleurodesis (fusion of the pleura because of incessant pleural effusions). For this purpose, povidone iodine is equally effective and safe as talc, and may be preferred because of easy availability and low cost.
Technical
PVP is also used in many technical applications:
• as adhesive in glue stick and hot melts
• as special additive for batteries, ceramics, fiberglass, inks, inkjet paper and in the chemical-mechanical planarization process
• as emulsifier and disintegrates for solution polymerization
• as photoresist for cathode ray tubes (CRT)
• use in aqueous metal quenching
• for production of membranes, such as dialysis and water purification filters
• as a binder and complexation agent in agro applications such as crop protection, seed treatment and coating
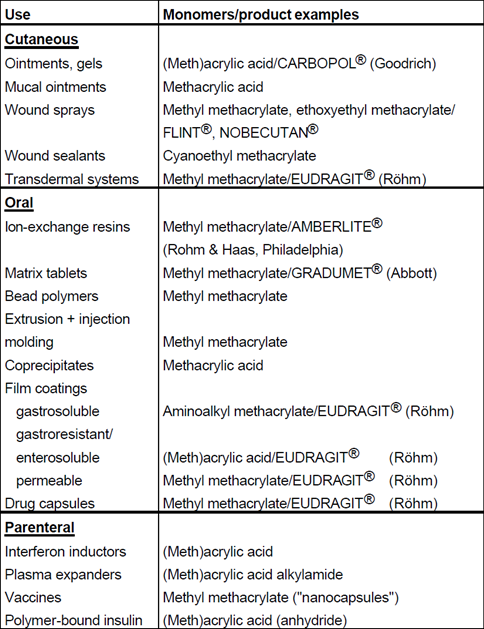
TABLE OF PHARMACEUTICAL APPLICATIONS

REFERENCES
1. E.T.Dunn, E.W.Grandmaison, M.F.A.Goosen. Applications and properties of chitosan.
2. Tozaki H, Odoriba T, Okada N, Fujita T, Terabe A, Suzuki T, Okabe S, Muranishi S, YamamotoA. Chitosan capsules for colon-specific drug delivery: enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J Controlled Release.2002;82 (1):51-61.
3. Jayvandan K. Patel, Rakesh P. Patel, Avani F Amin, Madhabhai M. Patel, Shree S.K. Patel, “Formulation and Evaluation of Mucoadhesive Glipizide Microspheres”, 2005, 1-4
4. Dyer A M, Hinchcliffe M, Watts P, Castile J, Jabbal-Gill I, Nankervis R, Smith A, Illum L, Nasal Delivery of Insulin Using Novel Chitosan Based Formulations: A Comparative Study in Two Animal Models Between Simple Chitosan Formulations and Chitosan Nanoparticles” Pharmaceutical Research, Vol. 19, No. 7, 2002, 998-1008. -140.
5. Anand Babu Dhanikula, Ramesh Panchagnula, “Development and Characterization of Biodegradable Chitosan Films for Local Delivery of Paclitaxel”, AAPS pharmatechnology, October 11, 6(3), 2004, 27.
6. Felt O, Baeyens V, Buri P, Gurny R, “Delivery of Antibiotics to the Eye Using a Positively Charged Polysaccharide as Vehicle”, AAPS PharmSci. 3 (4): 2001; 34.
7. Kamel A, Sokar M, Naggar V, Gamal S, “Chitosan and Sodium Alginate–Based Bioadhesive Vaginal Tablets” AAPS PharmSci, 4 (4): 2002; 44.
8. Nagwa H. Foda, Hanan M. Ellaithy, Mina I. Tadros, “Optimization of Biodegradable sponges as controlled release drug matrices. I. Effect of moisture level on chitosan sponge mechanical properties” Drug Development and Industrial Pharmacy, vol 30, No 4, Nov 2004, 369-379.
9. Aiedeh K, Taha MO. Synthesis of chitosan succinate and chitosan phthalate and their evaluation as suggested matrices in orally administered, colon-specific drug delivery systems. Arch Pharm (Weinheim). 1999;332(3):103-107.
10. Aiedeh K, Taha MO. Synthesis of iron-cross-linked chitosan succinate and iron-cross-linked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release beads. Eur J Pharm Sci. 2001;13(2):159-168.
11. National Toxicology Program Document. ntp-server.niehs.nih.gov/htdocs Chem_Background/ExSumPdf/Chitosan.pdf.
12. Hamman JH, Schultz CM, Kotze AF. N-trimethyl chitosan chloride.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

