About Authors:Kamal Sachdeva*2, Preeti Garg1, Manmohan Singhal2, Birendra Srivastava2
1. School of Pharmacy, Suresh Gyan vihar University, Jaipur
2. School of Pharmaceutical sciences,
Jaipur National University,
Jaipur, India
Abstract: The extracts of the stem bark of Jatropha curcas (Family-Euphorbiaceae) were tested for anti-diabetic activity, using blood glucose level in normal fasted rats, glucose tolerance test. Methanol: acetone: water (70:20:10) crude extract of Jatropha curcas at the dose of 100 and 300 mg/kg had shown significant protection and lowered the blood glucose levels to normal in glucose tolerance test. The extract showed a significant anti- diabetic activity comparable with that of glibenclamide. These results indicate that the Jatropha curcas stem bark possess significant anti – diabetic activity.
Reference Id: PHARMATUTOR-ART-1162
INTRODUCTION
Diabetes is a major degenerative disease in the world today, 1 affecting at least 15 million people and having complications which include hypertension, atherosclerosis and microcirculatory disorders.2 Diabetes mellitus is also associated with long-term complications, including retinopathy, nephropathy, neuropathy and angiopathy and several others.3 India has today become the diabetic capital of the world with over 20 million diabetics and this number is set to increase to 57 million by 2025.4 Diabetes mellitus (DM) is a multifactorial disease which is characterized by hyperglycemia,5 lipoprotein abnormalities,6 raised basal metabolic rate,7 defect in reactive oxygen species scavenging enzymes8 and high oxidative stress induced damage to pancreatic beta cells.9 Diabetes mellitus is ranked seventh among the leading causes of death and is considered third when it’s fatal complications are taken into account.10 Plants are well known in traditional herbal medicine for their hypoglycaemic activities, and available literature indicate that there are more than 800 plant species showing hypoglycaemic activity.11 There has been increasing demand for the use of plant products with antidiabetic activity due to low cost, easy availability and lesser side effects. Therefore, plant materials are continuously scrutinized and explored for their effect as hypoglycemic agents. Jatropha, a drought resistant shrub or tree, its wood and fruit can be used as for numerous purpose including fuels. It is used against mucosal diseases, arthritis, gout, jaundice, toothache, gum inflammation, gum bleeding. Plant extract used to treat allergies, burns, cuts and wounds, inflammation, leprosy, luekooderma, scabies and small-pox. Water extract of branches used in HIV, tumor, wound healing.12 The plant contains organic acid, cyclic triterpenes, stigmasterol,13 curcacycline A, curcin, a lectin Phobolesters Esterases, sitasterol and its d-glucoside.14 So this plant is studied for antidiabetic activity.
MATERIALS AND METHOD
Plant material and preparation of extract
Fresh stem bark of Jatropha curcas was collected from local area of Jaipur was identified in the department of botany, Rajasthan University, Jaipur. A voucher specimen number RUBL20844 was deposited in the department. The fresh stem bark was air dried to constant weight, pulverized. 200 gm powder of stem bark was subjected to soxhlet extraction with methanol: acetone: water (70:20:10). The extract was then filtered and concentrated to dryness. The extract was subjected to phytochemical test using reported methods.15, 16
Experimental Animals
Albino rats of either sex (150-200 g) were used for experimental purpose. The animals were housed in hygienic cages (6 rats / cage) under standard conditions of temperature (25±2)0C, relative humidity (45±20) % and (light) 12h: (dark) 12h cycle. The rats were fed with standard pellet diet (Amrut feeds, Chakan) and water ad libitum. The animals were allowed to acclimatize to experimental conditions by housing them for 8-10 days prior to the experiments. The experimental design and research plan along with animals handling and disposal procedure were approved by Institutional Animal Ethical Committee of Jaipur National University (1054/ac/07/CPCSEA) and IAEC approval number was JNU/IAEC/2010/02.
Acute toxicity studies
The acute toxicity was performed according to OECD guidelines (OECD 423, 2001). The selected female albino rats were used for toxicity studies. The animals were divided into four groups of three in each. The animals were fasted overnight prior to the acute experimental procedure. Extract was given orally to rats at the graded dose like 100, 300, 1000 and 2000 mg/kg Body Wt. Immediately, after dosing, the animals were observed continuously for first four hours for behavioral changes and for mortality at the end of 24 h, and daily up to 14 days for any behavioural change or mortality.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Anti-diabetic activity
Blood glucose level in normal fasted rats
Four randomized groups six in each of normal fasted rats were administered orally 150 mg & 300 mg/kg of the JCMSE, 0.6 mg/kg of glibenclamide and vehicle respectively. Subsequently, blood sugar levels were assessed at 1 h, 2 h and 3 h intervals.17
Glucose tolerance test
Four groups of 6 rats each were used for the study. Group 1 served as normal Vehicle, group 2 animals were administered with 0.6 mg/kg of glibenclamide orally, group 3 animals were administered with JCMSE 150 mg/kg orally and group 4 animals were administered with JCMSE 300 mg/kg orally. The rats of all the groups were loaded with 60% glucose (3 g/kg p.o.) 30 min after extract administration. Blood sugar levels were assessed at 1 h, 2 h and 3 h interval.18
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). Statistical comparisons were performed by one-way ANOVA followed by Dunnett's Multiple Comparison Test and the values were considered statistically significant.
RESULTS
In present investigation, antidiabetic activity of JCMSE at doses of 100 and 300 mg/kg, p.o. was evaluated using blood glucose level in normal fasted rats, glucose tolerance test.
Oral glucose tolerance test
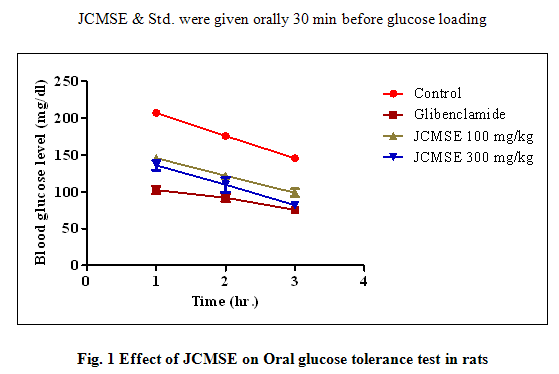
Experimental induction of hyperglycemia by intragastric ingestion of glucose resulted in a 1.5 to 2-fold increase in plasma glucose levels. The JCMSE 100 mg/kg, JCMSE 300 mg/kg and glibenclamide p.o. exhibited remarkable blood glucose lowering effect in the glucose tolerance test (Table 1) compare to control. Most significant reduction was observed for extract at the dose of 300 mg/kg on 2 hr. Glibenclamide showed reduction of blood glucose level at 1 hr., 2 hr. and 3 hr. respectively.
Table 1 - Effect of JCMSEon Oral glucose tolerance test in rats
|
Group |
Treatment |
Blood glucose level (mg/dl) |
|||
|
Fasting |
1 hr. |
2 hr. |
3 hr. |
||
|
A |
Glucose (3 gm.) |
76.95 ± 3.80 |
207.12 ±2.92 |
175.7 3±1.73 |
145.53 ±2.36 |
|
B |
Std.(0.6mg/kg) + Glucose |
69.23 ±5.27 |
102.37 ±5.05*** |
91.89 ±5.41*** |
75.25 ±3.69*** |
|
C |
JCMSE(100mg/kg) + Glucose |
71.86 ±5.00 |
145.76 ±4.56** |
121.83 ±3.51* |
98.64 ±5.12* |
|
D |
JCMSE(300mg/kg) + Glucose |
68.08 ±5.32 |
136.04 ±6.66*** |
109.71 ±9.97** |
81.69 ±2.14** |
Values are expressed as mean ± S.E.M. (n=/6). * P</0.05, ** P< 0.01, *** P<0.001 as compared to control. Two way Anova followed by Dunnett’s multiple comparison test.
DISCUSSION
Pancreas is the primary organ involved in sensing the organism’s dietary and energetic states via glucose concentration in the blood and in response to elevated blood glucose, insulin is secreted.2 Insulin deficiency leads to various metabolic alterations in the animals viz increased blood glucose, increased cholesterol, increased levels of alkaline phosphate and transaminases.19, 20 The literature review indicated hepatoprotective property of plant extract,21 the improvement of liver function and subsequent increase in uptake of blood glucose and its utilization may be another mechanism of action of the extract. Other possible mechanism includes the stimulation of β-cells and subsequent release of insulin and activation of the insulin receptors. Estimation of insulin level and insulin receptor may give more insight into the mechanism of the anti-diabetic activity exhibited by the extract.
A study in our laboratory showed that the crude extract of Jatropha curcas at the dose of 100 and 300 mg/kg reduced blood sugar significantly in normoglycemic rats and produced more intense hypoglycemia in the diabetic rats. In the present study, among the extracts are relatively more active; it can be suggested that the mode of action of the active constituent(s) of is probably mediated by an enhanced secretion of insulin, like biguanides.
The literature reports reveal that flavonoids and tannins present in the plant extract known to possess antidiabetic activity. In the present investigation also the observed antidiabetic potential of test extract may be due to presence of similar phytoconstitutes which was evident by preliminary phytochemical screening.
CONCLUSION
From this study, we can state that the extract of Jatropha curcas has beneficial effects on blood glucose levels. Further pharmacological and biochemical investigations will clearly elucidate the mechanism of action and will be helpful in projecting this plant as an therapeutic target in diabetes research.
REFERENCES
1. Ogbonnia SO, Odimegwu JI, Enwuru VN, Evaluation of hypoglycemic and hypolipidemic effects of ethanolic extracts of Treculia africana Decne and Bryophyllum pinnatum Lam. and their mixture on streptozotocin (STZ) - induced diabetic rats, African Journal of Biotechnology, 7(15), 2008, 2535-2539.
2. Edem DO, Hypoglycemic Effects of Ethanolic Extracts of Alligator Pear Seed (Persea Americana Mill) in Rats, European Journal of Scientific Research, 33(4), 2009, 669-678.
3. Kristova V, Liskoya S, Sotnikova S, Vojtko R, KurtanskyA, Sulodexide improves Endothelial
Dysfunction in Streptozotocin- Induced Diabetes in Rats, Physiol. Res., 5, 2008, 491-494.
4. Sridhar GR, Diabetes in India: Snapshot of a panorama, Current Sci., 83, 2000, 791.
5. Ugochukwu NH, Babady NE, Cobourne M, Gasset SR,The effect of Gangronema latifolium extracts on serum lipid profile and oxidative stress in hepatocytes of diabetic rats, Journal of Biosciences 28 (1), 2003,1-8.
6. Scoppola A, Montecchi FR, Mezinger G, Lala A, Urinary mevalonate excretion rate in type 2 diabetes: role of metabolic control, Atherosclerosis, 156, 2001, 357-361.
7. Owu DU, Antai AB, Udofia KH, Obembe AO, Obasi KO, Eteng MU, Vitamin C improves basal metabolic rate and lipid profile in alloxan-induced diabetes mellitus in rats, J. Biosciences 31(5), 2006, 575-579.
8. Kesavulu MM, Giri R, Kameswara RB, Apparao C, Lipid peroxidation and antioxidant enzyme levels in type 2 diabetic with microvascular complications, Diabetic Metabol, 26, 2000, 387 - 392.
9. Nayeemunnisa A, Alloxan diabetes-induced oxidative stress and impairment of oxidative defense system in rat brain: neuroprotective effects of cichorium intybus, Int J Diabetes & Metabolism, 17, 2009, 105-109.
10. Trivedi NA, Majumder B, Bhatt JD, Hemavathi KG, Effect of Shilajit on blood glucose and lipid profile in alloxan– induced diabetic rats, Indian J Pharmacol, 36, 2004, 373-76.
11. Rajagopal K, Sasikala K, Antihyperglycaemic and antihyperlipidaemic effects of Nymphaea stellata in alloxan-induced diabetic rats, Singapore Med J, 49, 2008, 137-141.
12. Heller J, Physic nut, Jatropha curcas, promoting the conservation and use of underutilized and neglected crops, International plant genetic resources institute, Rome, 1996, 66.
13. Khafagy SM, Mohamed YA, Abdel NA, Mahmoud ZF, phytochemical study of Jatropha curcas, Plant med, 31, 1977, 274-277.
14. Gubitz GM, Mittelbach M, Trabi M, Biofuels and industrial products from Jatropha curcas, DBV Graz, 97, 1997, 82-86.
15. Khandelwal KR, Practical pharmacognosy, edition 9th, Nirali Prakashan, Pune, 2002, 149-156.
16. Ansari SH, Essential of Pharmacognosy, edition 1st, Published by Birla Publication, New Delhi, 2005-2006, 588-93.
17. Du Vigneaud V, Karr WG, Carbohydrate utilization, rat of disappearance of D-glucose from the blood, J. Bio Chem., 66, 1925, 281-300.
18. Akhtar MA, Rashid M, Ibne Wahed MI, Islam MR, Shaheen SM, Islam MA, Amran MS, Ahmed M, Comparison of long-term antihyperglycemic and hypolipidemic effects between Coccinia cordifolia (Linn.) and Catharanthus roseus (Linn.) in alloxaninduced diabetic rats”, Res. J of Med. and Medical Sci., 2(1), 2007, 29-34.
19. Shanmugasundaram KR, Panneerselvam SP, Shanmugasundaram ERB, Enzyme changes and glucose utilization in diabetic rabbit,: The effect of Gymnema sylvestrae, R. Br. J. Ethnopharmacol, 7, 1983, 205-216..
20. Begum N, Shanmugasudnaram KR, Tissue phosphates in experimental diabetes, Arogya: J. Health Sci., 4, 1978, 129-139.
21. Mandal SC, Maity TK, Das J, Pal M, Saha BP, Hepatoprotective activity of Ficus racemosa leaf extract on liver damage caused by carbon tetrachloride in rat, Phytotherapy Research, 13(5), 1999, 430-32.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

