About Authors:
Ranabir Chanda, Saumya Samanta*, Dilip De, Jyotishman Bhattacharya, Anurup Mandal
Bengal School of Technology, Hooghly, West Bengal, India
*samantasaumya7@gmail.com
Abstract
Buccoadhesives have long been employed to improve the bioavailability of drugs undergoing significant hepatic first pass metabolism. Within the oral mucosal cavity, the buccal region offers an attractive route of administration for systemic drug delivery. The mucosa has a rich blood supply and it is relatively permeable. It is the objective of this article to review buccal drug delivery by discussing the structure and environment of the oral mucosa, mechanism of buccoadhesion, factors affecting buccoadhesion, polymers used ,types of buccal dosage form and the experimental methods used in assessing buccal drug permeation/absorption.
REFERENCE ID: PHARMATUTOR-ART-1689
Introduction
Amongst the various routes of drug delivery, oral route is the most preferred to the patient. However, disadvantages such as hepatic first pass metabolism and enzymatic degradation within the GI tract limits its use for certain drugs. Different absorptive mucosas are considered as potential site for drug administration eg, nasal, rectal, vaginal, ocular and oral cavity [1].
Bioadhesion may be defined as the state in which two materials, at least one of which is of biological nature, are held together for extended periods of time by interfacial forces [2]. For drug delivery purposes, the term bioadhesion implies attachment of a drug carrier system to a specific biological location. The biological surface can be epithelial tissue or the mucous coat on the surface of a tissue. If adhesive attachment is to a mucous coat, the phenomenon is referred to as mucoadhesion. Mucous coat includes the mucosal linings of the nasal, rectal, esophageal, vaginal, ocular, and oral cavity [3].
The idea of bioadhesive drug delivery systems was introduced as a new concept to the pharmaceutical sciences by the pioneering work of several research groups in the United States, Japan and Europe in the mid-1980s [4-7].
Since then, the idea to “stick” dosage forms to the site of application and/or drug absorption, respectively, has stimulated researchers all over the world. Originally, the advantages of bioadhesive drug delivery systems were seen in their potential (i) to prolong the residence time at the site of drug absorption (e.g., to reduce the dosing frequency for bioadhesive controlled release formulations) and (ii) to intensify contact with the underlying mucosal epithelial barrier (e.g., to enhance the epithelial transport of usually poorly absorbed drugs, such as peptides and proteins). The tight and close contact of drug delivery system (DDS) with the absorptive mucosa should generate a steeper concentration gradient, thus increasing the absorption rate [8].This principle, in particular, supported hopes of increased bioavailability of peptide drugs.
Later, it was discovered that some mucoadhesive polymers can also modulate the permeability of epithelial tissues by loosening the tight intercellular junctions [9-10]. and that some mucoadhesive polymers can also act as inhibitors of proteolytic enzymes [11-12].
These drug delivery system utilize property of bioadhesion of certain water soluble polymers which become adhesive on hydration and hence can be used for targeting particular site. Buccal delivery is the administration of the drug via buccal mucosa (lining of the cheek) to the systemic circulation [13].
1. Overview of the Oral Mucosa
1.1.Structure
The oral mucosa is composed of an outermost layer of stratified squamous epithelium (Figure 1). Below this lies a basement membrane, a lamina propria followed by the submucosa as the innermost layer. The epithelium is similar to stratified squamous epithelia found in the rest of the body in that it has a mitotically active basal cell layer, advancing through a number of differentiating intermediate layers to the superficial layers, where cells are shed from the surface of the epithelium [14].
The epithelium of the buccal mucosa is about 40-50 cell layers thick, while that of the sublingual epithelium contains somewhat fewer. The epithelial cells increase in size and become flatter as they travel from the basal layers to the superficial layers.
The turnover time for the buccal epithelium has been estimated at 5-6 days, and this is probably representative of the oral mucosa as a whole. The oral mucosal thickness varies depending on the site: the buccal mucosa measures at 500-800 µm, while the mucosal thickness of the hard and soft palates, the floor of the mouth, the ventral tongue, and the gingivae measure at about 100-200 µm. The composition of the epithelium also varies depending on the site in the oral cavity. The mucosa of areas subject to mechanical stress (the gingivae and hard palate) are keratinized similar to the epidermis. The mucosa of the soft palate, the sublingual, and the buccal regions, however, are not keratinized [15]. The keratinized epithelia contain neutral lipids like ceramides and acylceramides which have been associated with the barrier function. These epithelia are relatively impermeable to water. In contrast, non-keratinized epithelia, such as the floor of the mouth and the buccal epithelia, do not contain acylceramides and only have small amounts of ceramide [16-18].

Figure 1
1.2.Permeability
The oral mucosa in general is somewhat leaky epithelia intermediate between that of the epidermis and intestinal mucosa. It is estimated that the permeability of the buccal mucosa is 4-4000 times greater than that of the skin [19].
As indicative by the wide range in this reported value, there are considerable differences in permeability between different regions of the oral cavity because of the diverse structures and functions of the different oral mucosa. In general, the permeabilities of the oral mucosa decrease in the order of sublingual greater than buccal, and buccal greater than palatal. This rank order is based on the relative thickness and degree of keratinization of these tissues, with the sublingual mucosa being relatively thin and non-keratinized, the buccal thicker and non-keratinized, and the palatal intermediate in thickness and keratinized. [20]
It is currently believed that the permeability barrier in the oral mucosa is a result of intercellular material derived from the so-called ‘membrane coating granules’ (MCG) [21]. When cells go through differentiation, MCGs start forming and at the apical cell surfaces they fuse with the plasma membrane and their contents are discharged into the intercellular spaces at the upper one third of the epithelium. This barrier exists in the outermost 200µm of the superficial layer. Permeation studies have been performed using a number of very large molecular weight tracers, such as horseradish peroxidase. When applied to the outer surface of the epithelium, these tracers penetrate only through outermost layer or two of cells. When applied to the submucosal surface, they permeate up to, but not into, the outermost cell layers of the epithelium. According to these results, it seems apparent that flattened surface cell layers present the main barrier to permeation, while the more isodiametric cell layers are relatively permeable. In both keratinized and non-keratinized epithelia, the limit of penetration coincided with the level where the MCGs could be seen adjacent to the superficial plasma membranes of the epithelial cells. Since the same result was obtained in both keratinized and non-keratinized epithelia, keratinization by itself is not expected to play a significant role in the barrier function [22].
1.3.Environment
The cells of the oral epithelia are surrounded by an intercellular ground substance, mucus, the principle components of which are complexes made up of proteins and carbohydrates. These complexes may be free of association or some maybe attached to certain regions on the cell surfaces. This matrix may actually play a role in cell-cell adhesion, as well as acting as a lubricant, allowing cells to move relative to one another [23]. Along the same lines, the mucus is also believed to play a role in bioadhesion of mucoadhesive drug delivery systems [6]. In stratified squamous epithelia found elsewhere in the body, mucus is synthesized by specialized mucus secreting cells like the goblet cells, however in the oral mucosa, mucus is secreted by the major and minor salivary glands as part of saliva. Up to 70% of the total mucin found in saliva is contributed by the minor salivary glands [24].
At physiological pH the mucus network carries a negative charge (due to the sialic acid and sulphate residues) which may play a role in mucoadhesion. At this pH mucus can form a strongly cohesive gel structure that will bind to the epithelial cell surface as a gelatinous layer [14].
Another feature of the environment of the oral cavity is the presence of saliva produced by the salivary glands. Saliva is the protective fluid for all tissues of the oral cavity. It protects the soft tissues from abrasion by rough materials and from chemicals. It allows for the continuous mineralisation of the tooth enamel after eruption and helps in remineralisation of the enamel in the early stages of dental caries [25].
Saliva is an aqueous fluid with 1% organic and inorganic materials. The major determinant of the salivary composition is the flow rate which in turn depends upon three factors: the time of day, the type of stimulus, and the degree of stimulation. The salivary pH ranges from 5.5 to 7 depending on the flow rate. At high flow rates, the sodium and bicarbonate concentrations increase leading to an increase in the pH. The daily salivary volume is between 0.5 to 2 liters and it is this amount of fluid that is available to hydrate oral mucosal dosage forms. A main reason behind the selection of hydrophilic polymeric matrices as vehicles for oral transmucosal drug delivery systems is this water rich environment of the oral cavity [23].
[adsense:468x15:2204050025]
2. Mechanisms of Mucoadhesion
The mechanism of mucoadhesion is generally divided into two steps: the contact stage and the consolidation stage (Figure 2). The first stage is characterized by the contact between the mucoadhesive and the mucus membrane, with spreading and swelling of the formulation, initiating its deep contact with the mucus layer.
In the consolidation stage (Figure 2), the mucoadhesive materials are activated by the presence of moisture. Moisture plasticizes the system, allowing the mucoadhesive molecules to break free and to link up by weak van der Waals and hydrogen bonds [26].

Figure 2
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.Mucoadhesion Theories
Mucoadhesion is a complex process and numerous theories have been proposed to explain the mechanisms involved. These theories include mechanical interlocking, electrostatic, diffusion interpenetration, adsorption and fracture processes.
3.1. Wetting theory
The wetting theory applies to liquid systems which present affinity to the surface in order to spread over it. This affinity can be found by using measuring techniques such as the contact angle. The general rule states that the lower the contact angle, the greater is the affinity (Figure 3). The contact angle should be equal or close to zero to provide adequate spreadability. The spreadability coefficient, S AB0, can be calculated from the difference between the surface energies γB and γA and the interfacial energy γAB, as indicated in the equation given below. This theory explains the importance of contact angle and reduction of surface and interfacial energies to achieve good amount of mucoadhesion.
SAB = γB - γA - γAB

Figure 3
3.2. Diffusion theory
Diffusion theory describes the interpenetration of both polymer and mucin chains to a sufficient depth to create a semi-permanent adhesive bond (Figure 4). It is believed that the adhesion force increases with the degree of penetration of the polymer chains. This penetration rate depends on the diffusion coefficient, flexibility and nature of the mucoadhesive chains, mobility and contact time. According to the literature, the depth of interpenetration required to produce an efficient bioadhesive bond lies in the range 0.2-0.5 μm. This interpenetration depth of polymer and mucin chains can be estimated by the following equation:
l = (tD b)½
where t is the contact time and D b is the diffusion coefficient of the mucoadhesive material in the mucus. The adhesion strength for a polymer is reached when the depth of penetration is approximately equivalent to the polymer chain size. In order for diffusion to occur, it is important that the components involved have good mutual solubility, that is, both the bioadhesive and the mucus have similar chemical structures. The greater the structural similarity, the better is the mucoadhesive bond [27].
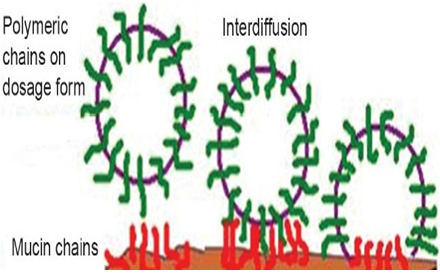
Figure 4
3.3. Fracture theory
This is perhaps the most used theory in studies on the mechanical measurement of mucoadhesion. It analyzes the force required to separate two surfaces after adhesion is established. This force, Sm, is frequently calculated in tests of resistance to rupture by the ratio of the maximal detachment force, Fm, and the total surface area, A0 , involved in the adhesive interaction.
Sm=Fm/Ao
Since the fracture theory (Figure 5) is concerned only with the force required to separate the parts, it does not take into account the interpenetration or diffusion of polymer chains. Consequently, it is appropriate for use in the calculations for rigid or semi-rigid bioadhesive materials, in which the polymer chains do not penetrate into the mucus layer [26, 27].
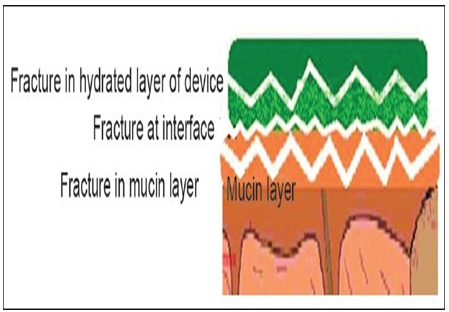
Figure 5
3.4. Eelectronic theory
This theory describes adhesion occurring by means of electron transfer between the mucus and the mucoadhesive system, arising through differences in their electronic structures. The electron transfer between the mucus and the mucoadhesive results in the formation of double layer of electrical charges at the mucus and mucoadhesive interface. The net result of such a process is the formation of attractive forces within this double layer [28].
3.5.Adsorption theory
In this instance, adhesion is the result of various surface interactions (primary and secondary bonding) between the adhesive polymer and mucus substrate. Primary bonds due to chemisorptions result in adhesion due to ionic, covalent and metallic bonding, which is generally undesirable due to their permanency [29].
Secondary bonds arise mainly due to van der Waals forces, hydrophobic interactions and hydrogen bonding. Whilst these interactions require less energy to "break", they are the most prominent form of surface interaction in mucoadhesion processes as they have the advantage of being semi-permanent bonds [30].
All these numerous theories should be considered as supplementary processes involved in the different stages of the mucus/substrate interaction, rather than individual and alternative theories. Each and every theory is equally important to describe the mucoadhesion process. There is a possibility that there will be initial wetting of the mucin, and then diffusion of the polymer into mucin layer, thus causing the fracture in the layers to effect the adhesion or electronic transfer or simple adsorption phenomenon that finally leads to the perfect mucoadhesion. The mechanism by which a mucoadhesive bond is formed will depend on the nature of the mucus membrane and mucoadhesive material, the type of formulation, the attachment process and the subsequent environment of the bond. It is apparent that a single mechanism for mucoadhesion proposed in many texts is unlikely for all the different occasions when adhesion occurs [31].
4.Factors affecting Mucoadhesion in the Oral Cavity
Mucoadhesive characteristics are a factor of both the bioadhesive polymer and the medium in which the polymer will reside. A variety of factors affect the mucoadhesive properties of polymers, such as molecular weight, flexibility, hydrogen bonding capacity, cross-linking density, charge, concentration, and hydration (swelling) of a polymer, which are briefly addressed below.
4.1. Polymer-related factors
4.1.1. Molecular weight
In general, it has been shown that the bioadhesive strength of a polymer increases with molecular weights above 100,000 8. As one example, the direct correlation between the bioadhesive strength of polyoxyethylene polymers and their molecular weights, in the range of 200,000 to 7,000,000, has been shown by Tiwari et al [32].
4.1.2. Flexibility
Bioadhesion starts with the diffusion of the polymer chains in the interfacial region. Therefore, it is important that the polymer chains contain a substantial degree of flexibility in order to achieve the desired entanglement with the mucus. A recent publication demonstrated the use of tethered poly(ethylene glycol)–poly(acrylic acid) hydrogels and their copolymers with improved mucoadhesive properties. The increased chain interpenetration was attributed to the increased structural flexibility of the polymer upon incorporation of poly(ethylene glycol). In general, mobility and flexibility of polymers can be related to their viscosities and diffusion coefficients, where higher flexibility of a polymer causes greater diffusion into the mucus network [33].
4.1.3. Hydrogen bonding capacity
Hydrogen bonding is another important factor in mucoadhesion of a polymer. It is said that in order for mucoadhesion to occur, desired polymers must have functional groups that are able to form hydrogen bonds. It is also confirmed that flexibility of the polymer is important to improve this hydrogen bonding potential. Polymers such as poly(vinyl alcohol), hydroxylated methacrylate, and poly(methacrylic acid), as well as all their copolymers, are polymers with good hydrogen bonding capacity [6].
4.1.4. Cross-linking density
The average pore size, the number average molecular weight of the cross-linked polymers, and the density of cross-linking are three important and interrelated structural parameters of a polymer network. Therefore, it seems reasonable that with increasing density of cross-linking, diffusion of water into the polymer network occurs at a lower rate which, in turn, causes an insufficient swelling of the polymer and a decreased rate of interpenetration between polymer and mucin.
4.1.5. Charge
Some generalizations about the charge of bioadhesive polymers have been made previously, where nonionic polymers appear to undergo a smaller degree of adhesion compared to anionic polymers. It is said that strong anionic charge on the polymer is one of the required characteristics for mucoadhesion. It has been shown that some cationic polymers are likely to demonstrate superior mucoadhesive properties, especially in a neutral or slightly alkaline medium. Additionally, some cationic high-molecular-weight polymers, such as chitosan, have shown to possess good adhesive properties.
4.1.6. Concentration
The importance of this factor lies in the development of a strong adhesive bond with the mucus, and can be explained by the polymer chain length available for penetration into the mucus layer. When the concentration of the polymer is too low, the number of penetrating polymer chains per unit volume of the mucus is small, and the interaction between polymer and mucus is unstable. In general, the more concentrated polymer would result in a longer penetrating chain length and better adhesion. However, for each polymer, there is a critical concentration, above which the polymer produces an “unperturbed” state due to a significantly coiled structure. As a result, the accessibility of the solvent to the polymer decreases, and chain penetration of the polymer is drastically reduced. Therefore, higher concentrations of polymers do not necessarily improve and, in some cases, actually diminish mucoadhesive properties. One of the studies addressing this factor demonstrated that high concentrations of flexible polymeric films based on polyvinylpyrrolidone or poly(vinyl alcohol) as film-forming polymers did not further enhance the mucoadhesive properties of the polymer. On the contrary, it decreased the desired strength of mucoadhesion [34].
4.1.7. Hydration (swelling)
Hydration is required for a mucoadhesive polymer to expand and create a proper “macromolecular mesh” of sufficient size, and also to induce mobility in the polymer chains in order to enhance the interpenetration process between polymer and mucin. Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding and/or electrostatic interaction between the polymer and the mucous network. However, a critical degree of hydration of the mucoadhesive polymer exists where optimum swelling and bioadhesion occurs [6].
4.2. Environmental factors
The mucoadhesion of a polymer not only depends on its molecular properties, but also on the environmental factors adjacent to the polymer. Saliva, as a dissolution medium, affects the behavior of the polymer. Depending on the saliva flow rate and method of determination, the pH of this medium has been estimated to be between 6.5 and 7.5. The residence time of dosage forms is limited by the mucin turnover time, which has been calculated to range between 47 and 270 min in rats 18 and 12–24 h in humans [35].
Movement of the buccal tissues while eating, drinking, and talking, is another concern which should be considered when designing a dosage form for the oral cavity. Movements within the oral cavity continue even during sleep, and can potentially lead to the detachment of the dosage form. Therefore, an optimum time span for the administration of the dosage form is necessary in order to avoid many of these interfering factors [36].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
5.Buccal Routes of Drug Absorption
The are two permeation pathways for passive drug transport across the oral mucosa: paracellular and transcellular routes. Permeants can use these two routes simultaneously, but one route is usually preferred over the other depending on the physicochemical properties of the diffusant. Since the intercellular spaces and cytoplasm are hydrophilic in character, lipophilic compounds would have low solubilities in this environment. The cell membrane, however, is rather lipophilic in nature and hydrophilic solutes will have difficulty permeating through the cell membrane due to a low partition coefficient. Therefore, the intercellular spaces pose as the major barrier to permeation of lipophilic compounds and the cell membrane acts as the major transport barrier for hydrophilic compounds. Since the oral epithelium is stratified, solute permeation may involve a combination of these two routes. The route that predominates, however, is generally the one that provides the least amount of hindrance to passage [20].
6. Buccal Mucosa as a Site for Drug Delivery
There are three different categories of drug delivery within the oral cavity (i.e., sublingual, buccal, and local drug delivery). Selecting one over another is mainly based on anatomical and permeability differences that exist among the various oral mucosal sites. The sublingual mucosa is relatively permeable, giving rapid absorption and acceptable bioavailabilities of many drugs, and is convenient, accessible, and generally well accepted [15].
The sublingual route is by far the most widely studied of these routes. Sublingual dosage forms are of two different designs, those composed of rapidly disintegrating tablets, and those consisting of soft gelatin capsules filled with liquid drug. Such systems create a very high drug concentration in the sublingual region before they are systemically absorbed across the mucosa. The buccal mucosa is considerably less permeable than the sublingual area, and is generally not able to provide the rapid absorption and good bioavailabilities seen with sublingual administration. Local delivery to tissues of the oral cavity has a number of applications, including the treatment of toothaches [37], periodontal disease [38,39], bacterial and fungal infections [40], aphthous and dental stomatitis [41], and in facilitating tooth movement with prostaglandins [42].
Even though the sublingual mucosa is relatively more permeable than the buccal mucosa, it is not suitable for an oral transmucosal delivery system. The sublingual region lacks an expanse of smooth muscle or immobile mucosa and is constantly washed by a considerable amount of saliva making it difficult for device placement. Because of the high permeability and the rich blood supply, the sublingual route is capable of producing a rapid onset of action making it appropriate for drugs with short delivery period requirements with infrequent dosing regimen. Due to two important differences between the sublingual mucosa and the buccal mucosa, the latter is a more preferred route for systemic transmucosal drug delivery [15,21].
First difference being in the permeability characteristics of the region, where the buccal mucosa is less permeable and is thus not able to give a rapid onset of absorption (i.e., more suitable for a sustained release formulation). Second being that, the buccal mucosa has an expanse of smooth muscle and relatively immobile mucosa which makes it a more desirable region for retentive systems used for oral transmucosal drug delivery. Thus the buccal mucosa is more fitted for sustained delivery applications, delivery of less permeable molecules, and perhaps peptide drugs.
Similar to any other mucosal membrane, the buccal mucosa as a site for drug delivery has limitations as well. One of the major disadvantages associated with buccal drug delivery is the low flux which results in low drug bioavailability. Various compounds have been investigated for their use as buccal penetration enhancers in order to increase the flux of drugs through the mucosa. Table 1 shows list of some buccal penetration enhancers.
Since the buccal epithelium is similar in structure to other stratified epithelia of the body, enhancers used to improve drug permeation in other absorptive mucosa have been shown to work in improving buccal drug penetration [43].
Drugs investigated for buccal delivery using various permeation/absorption enhancers range in both molecular weight and physicochemical properties. Small molecules such as butyric acid and butanol [44], ionizable low molecular weight drugs such as acyclovir [45,46], Propranolol [47], and salicylic acid [48], large molecular weight hydrophilic polymers such as dextrans [49], and a variety of peptides including octreotide [50], leutinizing hormone releasing hormone (LHRH) [51], insulin [43] and a-interferon [52] have all been studied.
A series of studies [49,53] on buccal permeation of buserelin and fluorescein isothiocyanate (FITC) labelled dextrans reported the enhancing effects of di- and tri-hydroxy bile salts on buccal penetration. Their results showed that in the presence of the bile salts, the permeability of porcine buccal mucosa to FITC increased by a 100-200 fold compared to FITC alone. The mechanism of penetration enhancement of FITC-labelled dextrans by sodium glycocholate (SGC) was shown to be concentration dependent [54].
Gandhi and Robinson [48] investigated the mechanisms of penetration enhancement of transbuccal delivery of salicylic acid. They used sodium deoxycholate and sodium lauryl sulfate as penetration enhancers, both of which were found to increase the permeability of salicylic acid across rabbit buccal mucosa. Their results also supported that the superficial layers and protein domain of the epithelium may be responsible for maintaining the barrier function of the buccal mucosa [20].
|
Permeation Enhancer |
Reference(s) |
|
Aprotinin |
55 |
|
Azone |
50, 56, 57 |
|
Benzalkonium chloride and Cetyltrimethylammonium bromide |
58 |
|
Cetylpyridinium chloride |
44, 58, 59, 60 |
|
Cyclodextrin |
52 |
|
23-lauryl ether , Dextran sulfate, Methoxysalicylate, Polyoxyethylene, Sodium EDTA and Sodium taurocholate |
61 |
|
Lauric acid, Menthol and Phosphatidylcholine |
62 |
|
Lauric acid/Propylene glycol |
43 |
|
Lysophosphatidylcholine |
63 |
|
Methyloleate and Oleic acid |
47 |
|
Polysorbate 80 |
59 |
|
Sodium glycocholate |
63, 64, 65 |
|
Sodium lauryl sulfate and Sodium salicylate |
55 |
|
Sodium taurodeoxycholate |
63 |
|
Various alkyl glycosides |
56 |
Table 1:
7. Techniques for the Determination of Mucoadhesion
The evaluation of bioadhesive properties is fundamental to the development of novel bioadhesive delivery systems. These tests are also important to screen large number of materials and their mechanisms. Numerous methods have been developed for studying mucoadhesion. Since no standard apparatus is available for testing bioadhesive strength, an inevitable lack of uniformity between test methods has arisen. Nevertheless, three main testing modes are recognized - tensile test, shear strength, and peel strength.
The most popular technique used for the determination of force of separation in bioadhesive testing is the application of force perpendicularly to the tissue/adhesive interface, during which a state of tensile stress is set up. But during the shear stress, the direction of the forces is reoriented so that it acts along the joint interface. In both tensile and shear modes, an equal pressure is distributed over the contact area [66].
The peel test is based on the calculation of energy required to detach the patch from the substrate. The peel test is of limited use in most bioadhesive systems. However, it is of value when the bioadhesive system is formulated as a patch [67].
In tensile and shear experiments, the stress is uniformly distributed over the adhesive joint, whereas in the peel strength stress is focused at the edge of the joint. Thus tensile and shear measure the mechanical properties of the system, whereas peel strength measures the resistant of the peeling force.
Review of the literature confirmed that the most common technique used for the measurement of bioadhesion test is tensile strength method. McCarron et al [67-69] and Donnelly [70] have reported extensively on the use of a commercial apparatus, in the form of a texture profile analyzer (Figure 6) operating in bioadhesive test mode, to measure the force required to remove bioadhesive films from excised tissue in vitro [71].

Figure 6
The texture analyzer, operating in tensile test mode and coupled with a sliding lower platform, was also used to determine peel strength of similar formulations (Figure 7) [67].
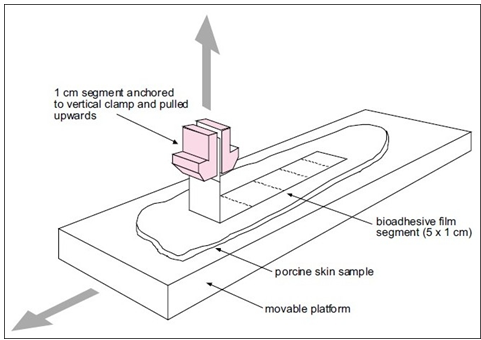
Figure 7
Rheological techniques that study the flow and deformation of materials may be useful in predicting the mucoadhesive ability of a polymeric formulation. A simple rheological approach for polymer solutions and gels was first suggested by Hassan and Gallo [72].
In this method, rheological interaction between a polymer gel and mucin solution was determined. It was shown that a polymer gel and mucin solution mixture exhibited larger rheological response than the sum of the values of polymer and mucin. However, a wide variation in results is found in the literature that utilize rheological methods for mucoadhesion determination, which may be attributable to differences in mucin type and concentration [73,74], as well as polymer concentrations [74,75]. Therefore, Hagerstrom [76] recommend that the rheological method should not be used as a stand-alone method for studying the mucoadhesive properties of the polymer gels.
In vivo aspects of mucoadhesive testing have recently been reported to monitor the mucoadhesion on tissue surface such as the GIT or the buccal cavity. However, there are only a limited number of in vivo studies reported in the literature in vitro work because of the time, cost, and ethical constrains. The most common in vivo techniques to monitor mucoadhesion include GI transit times of bioadhesive-coated particles and drug release from in situ bioadhesive devices.
Ch'ng [77] studied the in vivo transit time for bioadhesive beads in the rat GIT. A 51Cr-labeled bioadhesive was inserted at selected time intervals; the GITs were removed. The GIT of the rat was then cut into 20 equal segments and the radioactivity was measured.
Davis [78] investigated the noninvasive in vivo technique to determine the transit of mucoadhesive agent. Therefore, in this study a formulation was used containing a gamma-emitting radionuclide. The release characteristics and the position polymer could be examined by gamma scintigraphy.
In recent times, magnetic resonance imaging (MRI) is another noninvasive technique that is widely used. Christian Kremser [79] used MRI to detect the time and location of release of mucoadhesive formulation using dry Gd-DOTA powder.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
8.Experimental Methodology for Buccal Permeation Studies
Before a buccal drug delivery system can be formulated, buccal absorption/permeation studies must be conducted to determine the feasibility of this route of administration for the candidate drug. These studies involve methods that would examine in vitro and/or in vivo buccal permeation profile and absorption kinetics of the drug.
8.1.In vitro Methods
At the present time, most of the in vitro studies examining drug transport across buccal mucosa have used buccal tissues from animal models. Animals are sacrificed immediately before the start of an experiment. Buccal mucosa with underlying connective tissue is surgically removed from the oral cavity, the connective tissue is then carefully removed and the buccal mucosal membrane is isolated. The membranes are then placed and stored in ice-cold (4°C) buffers (usually Krebs buffer) until mounted between side-by-side diffusion cells for the in vitro permeation experiments. The most significant questions concerning the use of animal tissues as in vitro models in this manner are the viability and the integrity of the dissected tissue. How well the dissected tissue is preserved is an important issue which will directly affect the results and conclusion of the studies. To date, there are no standard means by which the viability or the integrity of the dissected tissue can be assessed. Dowty et al studied tissue viability by using ATP levels in rabbit buccal mucosa. Using ATP levels as an indicator for tissue viability is not necessarily an accurate measure, however. Dowty et al. reported a 50% drop in the tissue ATP concentration during the initial 6 hours of the experiment without a corresponding drop in tissue permeability. Despite certain gradual changes, the buccal tissue seems to remain viable for a rather long period of time. Therefore, a decrease in ATP levels does not assure a drop in permeability characteristics of the tissue. The most meaningful method to assess tissue viability is the actual permeation experiment itself, if the drug permeability does not change during the time course of the study under the specific experimental conditions of pH and temperature, then the tissue is considered viable [80].
Buccal cell cultures have also been suggested as useful in vitro models for buccal drug permeation and metabolism. However, to utilize these culture cells for buccal drug transport, the number of differentiated cell layers and the lipid composition of the barrier layers must be well characterized and controlled. This has not yet been achieved with the buccal cell cultures used thus far [81-84].
8.2. In vivo Methods
In vivo methods were first originated by Beckett and Triggs [85] with the so-called buccal absorption test. Using this method, the kinetics of drug absorption were measured. The methodology involves the swirling of a 25 ml sample of the test solution for up to 15 minutes by human volunteers followed by the expulsion of the solution. The amount of drug remaining in the expelled volume is then determined in order to assess the amount of drug absorbed. The drawbacks of this method include salivary dilution of the drug, accidental swallowing of a portion of the sample solution, and the inability to localize the drug solution within a specific site (buccal, sublingual, or gingival) of the oral cavity. Various modifications of the buccal absorption test have been carried out [86-89] correcting for salivary dilution and accidental swallowing, but these modifications also suffer from the inability of site localization. A feasible approach to achieve absorption site localization is to retain the drug on the buccal mucosa using a bioadhesive system [90-92].
Pharmacokinetic parameters such as bioavailability can then be calculated from the plasma concentration vs. time profile.
Other in vivo methods include those carried out using a small perfusion chamber attached to the upper lip of anesthetized dogs [93,94]. The perfusion chamber is attached to the tissue by cyanoacrylate cement. The drug solution is circulated through the device for a predetermined period of time and sample fractions are then collected from the perfusion chamber (to determine the amount of drug remaining in the chamber) and blood samples are drawn after 0 and 30 minutes (to determine amount of drug absorbed across the mucosa).
8.3. Experimental Animal Species
Aside from the specific methodology employed to study buccal drug absorption/permeation characteristics, special attention is warranted to the choice of experimental animal species for such experiments. For in vivo investigations, many researchers have used small animals including rats [43,44] and hamsters [57,95,96] or permeability studies. However, such choices seriously limit the value of the data obtained since, unlike humans, most laboratory animals have an oral lining that is totally keratinized. The rat has a buccal mucosa with a very thick, keratinized surface layer. The rabbit is the only laboratory rodent that has non-keratinized mucosal lining similar to human tissue and has been extensively utilized in experimental studies [61,97,98].
The difficulty in using rabbit oral mucosa, however, is the sudden transition to keratinized tissue at the mucosal margins making it hard to isolate the desired non-keratinized region [18].
The oral mucosa of larger experimental animals that has been used for permeability and drug delivery studies include monkeys [99], Dogs [88], and pigs [100-104].
Due to the difficulties associated with maintenance of monkeys, they are not very practical models for buccal drug delivery applications. Instead, dogs are much easier to maintain and considerably less expensive than monkeys and their buccal mucosa is non-keratinized and has a close similarity to that of the human buccal mucosa. Pigs also have non-keratinized buccal mucosa similar to that of human and their inexpensive handling and maintenance costs make them an equally attractive animal model for buccal drug delivery studies. In fact, the oral mucosa of pigs resembles that of human more closely than any other animal in terms of structure and composition . However, for use in in vivo studies pigs are not as ideal as dogs due to their rapid growth which renders the animal handling rather difficult. Miniature breeds of pigs can be used but their high cost is a deterrent. For in vitro studies though, because of easy availability and low cost porcine tissue is more suited as compared to dog buccal tissue [17,105].
9. Mucoadhesive Polymers used in Buccal Dosage Forms
Mucoadhesive polymers have numerous hydrophilic groups, such as hydroxyl, carboxyl, amide, and sulfate. These groups attach to mucus or the cell membrane by various interactions such as hydrogen bonding and hydrophobic or electrostatic interactions. These hydrophilic groups also cause polymers to swell in water and, thus, expose the maximum number of adhesive sites [106]. An ideal polymer for a bioadhesive drug delivery system should have the following characteristics [107,3]:
1. The polymer and its degradation products should be nontoxic and nonabsorbable.
- It should be nonirritant.
- It should preferably form a strong noncovalent bond with the mucus or epithelial cell surface.
- It should adhere quickly to moist tissue and possess some site specificity.
- It should allow easy incorporation of the drug and offer no hindrance to its release.
- The polymer must not decompose on storage or during the shelf life of the dosage form.
- The cost of the polymer should not be high so that the prepared dosage form remains competitive.
Polymers that adhere to biological surfaces can be divided into three broad categories [108,109]:
- Polymers that adhere through nonspecific, noncovalent interactions which are primarily electrostatic in nature
- Polymers possessing hydrophilic functional groups that hydrogen bond with similar groups on biological substrates
- Polymers that bind to specific receptor sites on the cell or mucus surface
The latter polymer category includes lectins and thiolated polymers. Lectins are generally defined as proteins or glycoprotein complexes of nonimmune origin that are able to bind sugars selectively in a noncovalent manner [110].
Lectins are capable of attaching themselves to carbohydrates on the mucus or epithelial cell surface and have been extensively studied, notably for drug-targeting applications [111,112].
These second-generation bioadhesives not only provide for cellular binding, but also for subsequent endo- and transcytosis. Thiolated polymers, also designated thiomers, are hydrophilic macromolecules exhibiting free thiol groups on the polymeric backbone. Due to these functional groups, various features of polyacrylates and cellulose derivatives were strongly improved [113].
The presence of thiol groups in the polymer allows the formation of stable covalents bonds with cysteine-rich subdomains of mucus glycoproteins leading to increased residence time and improved bioavailability [114].
Other advantageous mucoadhesive properties of thiolated polymers include improved tensile strength, rapid swelling, and water uptake behavior. Figure 8 shows the chemical structures of several bioadhesive polymers commonly used in modern drug delivery [71]. Table 2 shows classification of polymers used in buccal drug delivery system [115,116].

Figure 8
|
Criteria |
Catagories |
Examples |
|
Source |
Semi Natural |
Agarose, Chitosan, Gelatin, Hyaluronic Acid, Various Gums (guar, xanthan, gellan, carragenan, pectin and sodium alginate). |











.png)

