{ DOWNLOAD AS PDF }
About Authors:
Kambham Venkateswarlu1, N.Devanna2, N.B.L.Prasad3
1M.Pharm Scholar, Department Of Pharmaceutics,
2Director of JNTUA-Oil Technological Research Institute,
3Head of Examination branch-JNTUA-OTRI
k.v.reddy9441701016@gmail.com
Abstract:
The aim of the present study was to develop the microscopical authentication for the selected leaf of piper betel from the literature were the microscopical evidence is not available earlier and also to develop the pharmacognostic evaluation for the leaf of the crude drug and to evaluate the pharmacognostic parameters of the piper betel leaf. This work has clearly shown that the microscopical evidence for the leaf and what are the phytoconstituents present in the leaf.
I. INTRODUCTION
Piper betel (L. Piperaceae) leaves are widely used as a mouth freshener after meal. This plant is extensively grown in Bangladesh, India, Sri Lanka, Malaysia, Thailand, Taiwan and other Southeast Asian countries. Its common name is betel in English, paan in India and Bangladesh, phlu in Thailand and sirih in Bahasa Indonesian. Betel leaf is edible that has achieved an esteemed position in the human society right from the dawn of civilization, particularly in Bangladesh, Burma, China, India, Indonesia, Malaysia, Nepal, Pakistan, Philippines, South Africa, Sri Lanka, Thailand etc., where strong pungent aromatic flavoured leaves are traditionally used for chewing in their natural raw condition along with many other ingredients like sliced areca nut, slaked lime, coriander, aniseed, clove, cardamom, sweetener, coconut scrapings, ashes of diamond, pearl, gold and silver (Ayurvedic preparations), jelly, pepper mint, flavouring agent, fruit pulp etc. by the peoples.
Betel vine Piper betel (P. betel) belongs to the family of Piperaceae, popularly regarded as a medicinal plant in the South East Asia region. Experimentally, leaves of Piper betel are shown to possess antimicrobial [1, 12], antinociseptive & sedative activity [2], anti-inflammatory activity [10, 11, 13], anti-diabetic activity [4], antioxidant activity [5, 6, 7, 12, 13], analgesic activity [9, 11, 13], biological effects of some volatile oils [10], antipyretic activity[11]. The chief constituent of the leaves is a volatile oil which contains phenols, betel phenol, chavibetol and chavicol, cadinene and hydroxychavicol. The tribal population and aborigines of Bangladesh chew the leaves as a narcotic which causes swooning and profuse sweating and also helps to give warmth to the body during winter.
Medicinal plant have great value to phytochemists because of their medicinal properties, so that, the study of plants that have been traditionally used as pain killers should still be seen as a fruitful and logical research strategy in the search for new analgesic drugs and pain mechanisms.

Betel Plant cultivation in chittoor
Varieties include 'Magadhi' from Bihar, Gundi, Rasi & Bada varieties from Hinjilicut, Orissa which is more popular in Benaras, Mirzapur, Tunda, Agra & southern districts of Orissa, in India, and 'Venmony Vettila' from Kerala.
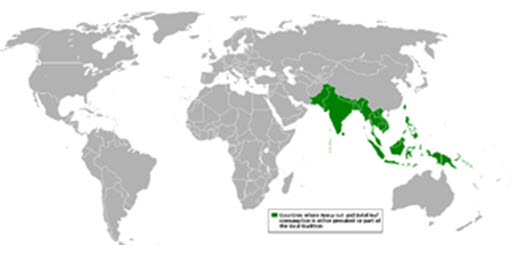
Betel leaf and cultivation in the world.
II. Methodology:
2.1. Plant collection:
The piper betel plant was collected around the Chittoor, in the Vengampalle village in the month of January-February. The whole plant collected was taken for authentication.
2.2. Pharmacognostic Evaluation:
2.2.1. Morphology of the leaf:
It refers to study of crude drugs by colour, odour, taste, size, shape and special features, like touch, texture etc.
2.2.2. Microscopy:
This microscopic study involves more detailed examination of a drug and it can be used to identify the organized drugs. The piper betel leaf microscopic study was focused towards
Leaf constants or Diagnostic characters of leaf:
1. Vein-islet number:
It is defined as the number of vein-islets per sq.mm of the leaf surface mid way between the mid rib and margin.
2. Vein-termination:
It is defined as the number of veinlet terminations per sq.mm of the leaf surface mid way between mid rib and margin.
3. Stomatal number:
It is average number of stomata per sq.mm of epidermis of the leaf.
4. Stomatal index:
It is the percentage which the number of stomata forms to the total number of epidermal cells; stomata being counted as one cell.
It is calculated by using the following equation.
S.I=S/E+S*100
Where S.I=stomata index
S= no. of stomata per unit area
E=no. of epidermal cells in the same unit area.
5. Stomata:
A stomata is minute epidermal opening present on Arial parts of the plants, with following characteristics
I. A central pore
II. Two kidney shaped similar cells containing known as guard cells and varying number of subsidiary cells covering the guard cells.
6. Trichomes: Trichomes are the hairy outergrowth of the epidermal cells of the leaf.
7. Tran version section of betel leaf:
The transverse section was carried out through the mid rib region. The arrangement of tissues in transverse and longitudinal sections and type of cells and cell contents are revealed by suitable histological study of a crude drug with the aid of microscope.
2.3. Powder microscopy:
The dried piper betel leafs are make a fine powder by using mortar pestle and pass it on the sieve. Then it is used for the tests.
2.3.1. Test for starch grains:
Take a pinch of betel leaf powder and to this add N/50 iodine solution and focus on the microscope. The hemicelluloses blue stain is present means it shows that presence of starch grains.
2.3.2. Test for calcium oxalate crystals:
In a watch glass take a pinch of betel leaf powder, to this add few ml of glycerine water and focus on the microscope. If it shows the yellow colour cubical shape crystals, it means presence of calcium oxalate crystals.
2.3.3. Test for lignified cells:
Take a pinch of betel leaf powder and to this add Phloroglucinol and concentrated hydrochloric acid and mount the sample in a slide and cover the slide with cover slip under observe under microscope for any lignified cells.
2.4. Preliminary Phytochemical Screening:
* Preparation of extracts:
100 gm of powdered material was cold macerated with distilled water (500ml) for 24 hours to obtain aqueous extract. Extract was filtered and concentrated by distilling of the solvent and then evaporates to dryness on the water bath. They were stored in desiccators until further use.
2.5. Qualitative Chemical Test:
The extract of piper betel was subjected to following chemical tests for the identification of the chemical groups present in them.
1. Detection of alkaloids:
A small quantity of the extract was treated with few drops of dil.HCL and filtered. The filtrate was used following tests.
Mayer’s test:
The filtrate was treated with the Mayer’s reagent. The cream colour precipitate was produced.
b.Dragendraff’s test:
The filtrate was treated with the Dragendraff’s reagent Orange brown precipitate was obtained.
2. Detection of flavonoids:
A.Shinoda test:
The extract was treated with 1gm of magnesium turnings and few drops of conc.hcl and boiled for 5 min. the formation of orange colour shows presence of flavonoids.
B. A small portion of each extracts was dissolved separately in alkali yellow colour was observed.
3. Detection of glycosides:
Test for cardiac glycosides:
a. Keller-Killani test:
Extracts were dissolved in 3 ml of glacial acetic acid and add to these 2 drops of ferric chloride. The contents are then transferred to a test tube containing 2 ml of conc.H2S04. Re dish brown colour was formed at the junction of two layers.
b. Legal’s test:
To the extracts add 1 ml of Sodium nitro prusside and NaoH. Colour changes from pink to red were observed.
* Test for Anthraquinone glycosides:
A.Borntrager test: about 1ml of extract was boiled with dil.H2SO4 and filtered. The filter ate was extracted with chloroform. The chloroform layer was separated and equal quantity of dil. Ammonia was added. Formation of pink or red colour in organic layer indicates presence of glycosides.
B. A little amount of solution was treated with 5N hypo sulphite. Appearance of red colour indicates presence of glycoside.
4. Detection of carbohydrates:
A minimum amount of various above extracts were taken and dissolved in the respective solvent and treated with hydrochloric acid to detect the presence of carbohydrates.
Molisch’s test:
The solution was treated with 2-3 drops of alcoholic alpha naphthol and 2 ml of conc. H2SO4 was added along sides of the test tube appearance of violet colour indicates the presence of carbohydrate.
Fehling’s test:
About 2 ml of the solution was treated with 1ml of Fehling’s solution and heat on the water bath. A reddish orange precipitate was produced to indicate the presence of carbohydrate.
5. Detection of proteins and amino acids:
A. Biuret test:
1ml of solution was treated with equal volume of 5%NaoH and 1% Cuso4 solution. A violet colour was produced.
B. Ninhydrin’s test:
1ml of solution was treated with ninhydrine reagent and heated, purple colour was produced.
6. Detection of Phytosterols:
A small amount of various extracts was dissolved in 5ml of chloroform separately. This solution was subjected to following tests.
A. Salkowski’s test:
To 1ml of the solution, few drops of conc.H2SO4 added brown colour was produced.
B. Libermann-Buchard’s test:
The prepared chloroform solution was treated with 2 drops of conc.H2S04 acid, Followed by 3 drops of acetic anhydride. Emerald green colour formed.
7. Detection of Saponins:
A. Foam test:
The extract was diluted with 20 ml of distilled water and agitated in a graduated cylinder for 15 minutes. Appearance of stable foam indicates the presence of Saponins.
B. Haemolysis test:
The extract was spreaded over the glass slide to form a thin layer on which adds a drop of human blood. Observe under microscope for a change in structure of R.B.C structure for haemolysis.
8. Detection of phenolic compounds:
A. Test for phenolic compounds:
To the extract, Dil. Ferric chloride was added, blue colour was formed.
B. Test for tannins
- To the extract 1% of gelatine and 10% of sodium chloride was added then a white precipitate was formed.
- The presence of white precipitate in the test solution, when treated with lead acetate solution indicates presence of tannins.
- On treated with Bramer’s reagent, it produces a brown precipitate.
9.Detection of fixed oils:
- A small quantity of extracted solution was pressed between two filter papers. An oil stain was found, of fixed oil was present.
- On treatment with Sudan red!!! Or tincture of alkanna produces red colour.
- On treatment with osmic acid, produces brown or black colour.
10.Detection of Gums and Mucilage:
- To the extract, Ruthenium red solution was added, the formation of pink colour indicates the presence of mucilage in the extract.
- With Methylene blue produces deep blue colour.
- With Iodine and sulphuric acid produces violet colour.
11. Detection of Aromatic acids:
To the small quantity of the solution, neutral ferric chloride was added; a buff colour precipitate was formed, if aromatic acid was present.
12. Test for triterpinoids:
The extract was treated with few drops of Antimony chloride; appearance of blue precipitate denotes the presence of triterpinoids.
III. RESULTS
3.1. Pharmacognostic Study:
3.1.1. The morphology of piper betel has been studied
Colour: Deep green colour
Shape: Heart shaped
Length: 15-18 cm long width: 10cm
Odour: Aromatic
Margin: Entire margin
Apex: Acute
Base: Symmetric base
3.1.2. Microscopy: The microscopy of piper betel leaf shows that
Vein-let number:
The total vein let numbers are present in the 1 sq mm were 32.
Vein-termination:
The vein terminations present in the 1 sq mm were 123.
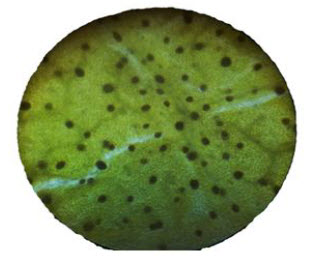
Fig.No.1.Vein terminations of the upper layer of the leaf

Fig.No.2.Surface view of betel leaf-morphology

Fig. No.3. Vein lets present in the upper surface of the leaf
Stomatal number:
The stomatas present in the 1 sq mm of the leaf were 32.
Stomata index:
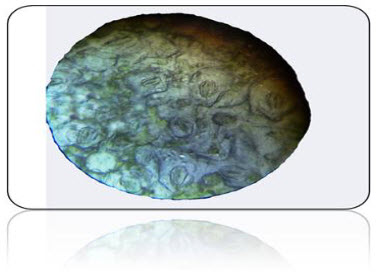
Fig .No.4.T.S. View of Stomata present in the upper epidermis
The stomata having the more than two guard cells- so it is Anamocytic type stomata
Trans version section of betel leaf:
* It shows that upper, lower epidermal cells and it gives pink colour stain with a drop of phloroglucinol and concentrated hydrochloric shows that presence of lignified cells like xylem and phloem in the vascular bundles of midrib region. And also present prismatic calcium oxalate crystals.
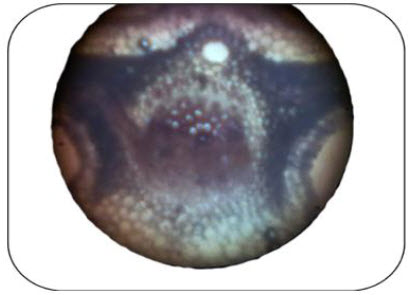
Fig. No.5.T.s view of betel leaf in the mid rib region
* Powder microscopy :the powder microscopy shows that presence of starch grains, calcium oxalate crystals and xylem, phloem
3.2. Preliminary Phytochemical Screening:
- Preliminary phytochemical screening: The qualitative chemical tests were performed for the P. betel dried powder according to the methods described by Farnsworth with some modifications.
- It shows that presence of alkaloids, tannins, phenolic compounds, flavonoids and steroid glycosides, terpens, anthraquinones, and steroids
- Terpenes or terpenoids have been identified as active antiprotozal and antimalarial agents in many pharmacological studies Flavonoids are the other forms of Piper betel phenolic structures. Flavonoids revealed significant anti-parasitic activities against different parasite strains of malaria, trypanosome and leis mania Derivatives of 9, 10-anthraquinone include many important drugs including antimalarials like rufigallol. These chemical compounds which were found in this extract may be acting singly or in synergy with one another to exert the observed antiplasmodial activity of Piper betel.
IV. Conclusion:
The present study reveals that the microscopically evidence was strong enough to prove the leaf and the phytochemical screening reports the presence of alkaloids, tannins, phenolic compounds, flavonoids and steroid glycosides, terpenes, anthraquinones, and steroids which may be responsible for various therapeutic activities.
So there is a lot of scope for in vivo studies due to presence of different phytoconstituents.
V. REFERENCES:
1.Agarwal T, Singh R, Shukla AD, Waris I, Gujrati A., Comparative analysis of antibacterial activity of four Piper betel varieties. Adv Appl Sc Res, 2012, 3: 698-705.
2.Ahmed F, Hussein MH, Rahman AA, Shahid IZ., Antinociceptive and sedative effects of the bark of Cerebra odollam Gareth. Orient Pharm Exp Med, 2006, 6: 344-348.
3.Antonio AM, Brito ARMS. Oral anti-inflammatory activity of a hydro alcoholic extract and partitioned reactions of Turnera ulmifolia (Turneraceae). J Ethnopharmacol, 1998, 61: 215- 228
4.Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD., antidiabetic activities of aqueous and ethanolic extracts of Piper betel leave in rats. J Ethnopharmacol, 2005, 102: 239-245.
5. Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I., Antioxidant principles from Bauhinia terapotensis. J Nat Prod, 2001, 64: 892–895
6. Choudhary D, Kale RK., Antioxidant and non toxic properties of Piper betel leaf extract: in vitro and in vivo studies. Phytother Res, 2002, 16: 461-466.
7.Dasgupta N, De B. Antioxidant activity of Piper betel L. leaf extract in vitro. Food Chem, 2004, 88: 219-224.
8. Elisabetsky E, Amador TA, Albuquerque RR, Nunes DS, Cavalho ACT., Analgesic activity of Psychotria colorata (Willd. Ex R. and S.) Muell. Arg. Alkaloids. J Ethnopharmacol, 1995, 48: 77-83.
9. Garg SC, Jain R., Biological activity of the essential oil of Piper betel L. J Essen Oil Res, 1992, 4: 601-606.
10. Gupta M, Mazumder UK, Gomathi P, Selvan VT., Anti-inflammatory evaluation of leaves of Plumeria acuminate. BMC Complementary and Alternative Medicine, 2006, 6: 36-39.
11.Gupta M, Mazumder UK, Kumar RS, Gomathi P, Rajeshwar Y, Kakoti BB, Selven VT., Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. Journal of Ethnopharmacology, 2005, 98: 267-273.
12.Hela AE, Abdullah A., Antioxidant and antimicrobial activities of methanol extracts of some Verbena species: In vitro evaluation of antioxidant and antimicrobial activity in relation to polyphenolic content. Journal of Applied Science Research, 2010, 6: 683-689.
13.Hassan MS, Alam MB, Chowhdury NS, Asadujjaman M, Zahan R, Islam MM, Mazumder MEH, Aqua ME, Islam A., Antioxidant, analgesic and anti-inflammatory activities of the herb Eclipta prostrata. J Pharmacol Toxicol, 2011, 6: 468-480.
REFERENCE ID: PHARMATUTOR-ART-2142
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 4 Received On: 05/02/2014; Accepted On: 25/02/2014; Published On: 01/04/2014 How to cite this article: K Venkateswarlu, N Devanna, NBL Prasad, Microscopical and Preliminary Phytochemical Screening of ‘Piper betel’, PharmaTutor, 2014, 2(4), 112-118 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

