About Authors:
Pankaj S. Waghere1*, Malpani Amol2,
1Prin. K. M. Kundnani College of Pharmacy, Mumbai. Maharashtra, India.
2Roland Institute of Pharmaceutical Sciences, Berhampur. Orissa, India.
*waghere.pankaj@gmail.com
ABSTRACT
Hydrocolloids are naturally-occurring plant polysaccharide, in that gaur gum is most useful and validated natural plant polysaccharide. Guar gum and their derivatives are widely used in pharmaceutical dosage forms. Many are used as biodegradable polymeric materials to deliver active pharmaceutical ingredients. Natural polymers can be modified to obtain tailor-made materials for drug delivery systems and to compete with the synthetic biodegradable excipients available in the market. Natural polymers as a drug carrier material are of two types: polysaccharides and proteins. They found both in plants and animals providing several advantages over synthetic polymers.Gaur gums are preferred to those of synthetic origin due to their green, cost-effectiveness, nontoxic, easy availability and for suitable binder in tablet manufacture. The aim of this review is to provide an insight into the many potential applications of gaur gum as pharmaceutical natural excipients.
Reference Id: PHARMATUTOR-ART-1359
1. INTRODUCTION
Natural polymers are defined as polymeric materials derived from plant exudates. In thousands of years back, most useful material in nature is natural polymer in various purpose for medicinal application but now a day new approach is the hydrophilic polymers as a plant source and they are derived in to three groups-
1) Natural polymers like polysaccharide
2) Semi-synthetic polymers like derived from cellulose
3) Synthetic polymers like based on vinyl and acrylic monomers.
Natural polymers A group of carbohydrate polymers of this class are obtained from plant exudates, gums, and extracts mainly include polysaccharides. They can absorb large quantities of water and swell. They find wide range of pharmaceutical applications that includes their use as binder, disintegrant in tablets, emulsifiers, suspending agents, and gelling agents and also as sustaining agents in solid dosages1.Pharmaceutical application most economically important are guar gum, having combination of galactose and mannose units.
Semi-syntheticpolymersImportant polymers in this class are known as cellulose ethers containing ethyl ether of cellulose where hydroxyl radicals of cellulose are replaced by ethoxy groups. Cellulose ethers are further classified into nonionic includes hydroxypropyl methylcellulose and anionic includes sodium carboxymethyl cellulose.
Synthetic polymersImportant polymers in this class are generally derived from vinyl and acrylic monomers. These polymers have a wide capacity to absorb water due to polar functional groups on their chain backbones. Some important synthetic polymers include cross-linked polyacrylamide, hydroxyethyl methacrylate, polyvinyl alcohol, polyacrylic acid, polyacrylate etc.
In recent years, considerable attention has been focused on hydrophilic polymers in Pharmaceutical sector, for example, its properties are of fundamental importance for the controlled release of drug in the gastrointestinal tract (GIT)2, as well as carrier for colon targeted for drug in the treatment of colorectal cancer3. The current demand for guar gum outstrips supply and guar being introduced into new emerging area. Taking into consideration that guar gum polymer is a cheap, easily available and non-toxic4. Gaur gum exhibits several favorable properties, such as biocompability and biodegradability5. Natural polysaccharides such as gaur gum are not digested in human stomach or small intestine, but are degraded in the colon by resident bacteria6. Guar gum is reported to be potential carriers for novel colon specific drug delivery7, 8. In pharmaceutical formulations, guar gum is used as a binder, disintegrant9-11 (see Table 1), suspending agent, thickening agent and stabilizing agent12, 13. Guar gum has been investigated as a potential adjuvant for swellable controlled drug delivery systems. Therapeutically, gaur gum has been used as part of the diet of patients with diabetes mellitus14, 15. It has also been used as an appetite suppressant in tablet form. Guar gum is new effective cancer chemopreventive and/or an anti-inflammatory activity has become an important worldwide strategy in cancer prevention16. It was also found to have the ability to bind toxic substances to carry them out of the body, and to significantly decrease the levels of blood sugar, cholesterol, triglycerides, and lipids in normal and diabetic rodents17, 18.
2. CHEMICAL STRUCTURE AND MANUFACTURING PROCESS OF NATURAL HYDROPHILIC POLYMER
2.1. Guar gum
2.1.1. Structure and properties
Guar bean have a large endosperm that contains galactomannan gum, a substance which forms a gel in water, commonly known as guar gum (Cyamopsis tetragonolobus, family Leguminosae).The gum consists of 85% guaran, a water soluble polysaccharide containing linear chains of (1→4)-β -D-mannopyranosyl units with α-D-galactopyranosyl units attached by (1→6) linkages19 (Figure. 1). The ratio of galactose to mannose is 1:2. Generally, galactomannans properties depend on their chemical structure, such as chain length, availability of cis-OH groups, steric hindrance, degree of polymerization and additional substitutions20 which resulted in considerable variations in their functional characteristics. Guar gum has five to eight times the thickening power of starch and has many uses in cosmetics, food product and pharmaceutical formulations. It was reported that guar gum was first used for food products in the United State in 194921. The largest market for guar gum (EU food additive code E412) is in the food industry. The most importance property as an ability to get hydrate rapidly in cold water to attain uniform and very high viscosity at relatively low concentrations. Apart from being the most effective emulsifier, thickener, binder, disintegrant and stabilizer decidesits application in the technology of a drug form. It is used in the production of ointments, hydrogels, for modification of solution viscosity, stabilization of emulsion and also as a binding agent in the production of tablets22.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
In addition to the use of guar gum in food and pharmaceuticals, its utility in combination with other excipients have been reported19 in which a mixture of guar gum, a bovine gelatin hydrolysate, sodium glycinate and appropriate amount of flavoring agents was blended and heated to yield a product which are administered orally for the treatment of digestive tract disorders23. Guar gum hydrates and swells rapidly in aqueous media to form viscous dispersions or gel layer on the tablet surface.Consequently, drug releases out from the guar gum tablet in a sustained release manner, achieving the desired kinetics.The current demand for guar gum introducing into new emerging area especially therapeutic and medicinal role as hypocholesterolemic and hypoglycemic agent24. Guar gum suitably inexpensive, commercially available and green (of natural origin) polymers for use as stabilizing agents for iron nanoparticles. It is most effective polymer for long-term stabilization of the nanoparticles25.
2.1.2. Manufacturing process
Depending upon the requirement of the end product, various techniques are used for processing guar gum.
Guar seed: The pods are sun dried, manually separated from the seeds and the seed are supplied to the industry for processing.
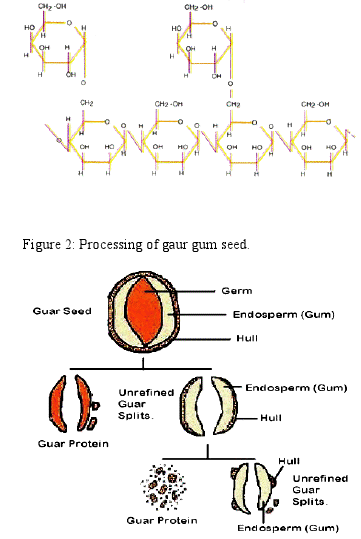
Undehusked Guar splits: A Mechanical process of roasting, differential attrition, sieving and polishing, commercially extracts the gum from the seeds. The seeds are broken and the germ is separated from the endosperm. Two halves of the endosperm are obtained from each seed and are known as undehusked Guar splits (Figure 2. ).
Refined Guar splits: Refined Guar splits are obtained when the fine layer of fibrous material, which forms the husk, is removed and separated from the endosperm halves by polishing.
Guar powder: The refined Guar splits are then treated and finished into powder by a variety of routes and processing techniques depending upon the desired end product.
2.1.3. Pharmacopeial Specifications26
The pharmaceutical requirement for guar gum include appearance, density, viscosity, loss on drying, heavy metal content are listed in Table 2.
2.1.4. Composition of guar gum17
Guar is simple, non-ionic and best characterized branched polysaccharide extracted from Cyamopsis tetragonoloba seeds. The compositions of guar gum are listed in Table 3.
2.1.5. Guar gum powder standard
HS-Code- 130 232 30
CAS No. - 9000-30-0
EEC No. - E 412
BT No. - 1302 3290
EINECS No. - 232-536-8
Imco Code- Harmless
2.1.6. Application of gaur gum in pharmaceutical formulation
2.1.6.1. Nanoparticulate Drug Delivery Systems (NDDS)
In recent years, the interest in sub-micron systems (i.e. nanosystems) in pharmacy has surged. This is, in part, due to the potential advantages these systems provide over more conventional microparticulate systems. Nanotechnology encompasses a wide array of sub-micron systems such as nanoparticles, nanocapsules, lipid complexes, polymeric micelles and dendrimers27.Guar gum was used as nanoparticulate drug delivery systems in the form of nanospheres and nanocapsules based on cross-linked guar gum that are capable of incorporating a higher amount of tamoxifen citrate with a relatively slower in vitro release profile and to demonstrate the efficacy of these systems in female albino mice.Guar gum nanospheres containing tamoxifen citrate were prepared and characterized for using it as a carrier for targeted drug delivery for treatment breast cancer28.
2.1.6.2. Colonic Drug Delivery Systems (CDDS)
Colonic drug delivery (CDD) has gained popularity in recent years, in part due to its being a potential absorption site for proteins and peptides29, 30. The colon is also potentially a good site for absorption of small molecules that are sensitive to the more enzymatically active and hostile environment of the gastrointestinal tract (GIT).
Guar gum has been extensively used for colon delivery31, 32 due to its drug release retarding property and susceptibility to microbial degradation in the large intestine.
A novel colon specific drug delivery system based on a polysaccharide, guar gum was evaluated in healthy human male volunteers, with γ-scintigraphic study using Technetium 99m-DTPA as tracer. It was seen that some amount of tracer present on the surface of the tablets was released in stomach and small intestine and the bulk of the tracer present in the tablet mass was delivered to the colon33. Other investigators evaluated guar gum-based matrix tablets that released only 5–10% of their drug content combined in the stomach and small intestine. However, the bacterial flora of the colon digested the gum releasing the entire drug content34. Guar gum containing polygalactomannans used as a carrier for colonic drug delivery. Matrix tablets of dexamethasone were evaluated for colon specific drug delivery using guar gum as a carrier35. Guar gum microspheres prepared and characterized for local release of drug in the colon which is effective treatment of colorectal cancer3.
The use of guar gum in colon targeted drug delivery in the form of matrix and compression coated tablets has also been reported36, 37.
2.1.6.3. Oral Drug Delivery Systems (ODDS)
Oral ingestion has long been the most convenient and commonly employed route of drug delivery due to its ease of administration, low cost and flexibility in the design of the dosage form. Guar gum is becoming very popular in formulating oral controlled release tablets. As the dissolution medium penetrates the dosage form, the polymer material swells and drug molecules begin to move out of the system by diffusion at a rate determined by the nature and composition of the polymer as well as formulation technology. Design oral controlled drug delivery systems for highly water-soluble drugs using guar gum as a carrier in the form of three-layer matrix tablets, trimetazidine dihydrochloride was chosen as a model drug because of its high water solubility. The three-layer guar gum matrix tablet provided the required release rate on par with the theoretical release rate for guar gum formulations meant for twice daily administration. The results indicated that guar gum, in the form of three-layer matrix tablets, is a potential carrier in the design of oral controlled drug delivery systems for highly water-soluble drugs such as trimetazidine dihydrochloride38. The same study was carried out by using metoprolol tartrate a model drug with high solubility. The results indicated that guar gum, in the form of three-layer matrix tablets, is a potential carrier in the design of oral controlled drug delivery systems for highly water-soluble drugs such as metoprolol tartrate39.
2.1.6.4. Transdermal Drug Delivery System (TDDS)
TDDS involves optimization of several factors such as release rate, stability, safety, convenience of use etc. TDDS formulations possess good films forming properties, should be non-irritating, inert, and stable. Carboxymethyl guar (CMGS), an anionic semi-synthetic guar gum derivative was evaluated for its suitability of use in TDDS40 and as supplementing agents to increase the viscosity for osteoarthritis treatments41.
2.1.6.5. Mucoadhesive Drug Delivery Systems (MDDS)
"Mucoadhesive" commonly used for materials that bind to the mucin layer of the biological membrane. Mucoadhesive polymers have been utilized in many different dosage forms in effort to achieve systemic delivery of drugs through different mucosae. MDDS include tablets, patches, tapes, films, semisolids and powders. Pharmaceutical buccal patch is the selection and characterizations of an appropriate bioadhesive in the formulation. The polymer that are commonly used as bioadhesive in the pharmaceutical applications are guar gum, acacia, chitosan, hydroxyethylcellulose, hydropropylcellulose, sodium alginate and poly (vinyl alcohol)42. The strong interaction between the polymer and the mucous lining of the tissue helps increase contact time and permit localization. Polymer like guar gum was tested by shear stress measurement and detachment force measurement methods43. In vitro bioadhesion strength study and optimize drug release profile by using a natural hydrophilic polymer such as guar gum and combinations of other polymer were used to formulate matrix tablets44.
FUTURE PROSPECTS
Natural source of polymer is gaining importance now days because of their several pros over synthetic polymers. Traditionally, inert excipients were used for fulfilling volume and for assisting the manufacturing process of formulation. Most of the new chemical entities introducing which have unfavorable physicochemical and pharmacokinetic properties, there polymers are widely used to improve drug delivery. Particularly, Guar gum and its derivatives have much potential to serve as an important excipient in various new drug delivery systems in future.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1. Uses of guar gum
|
Use |
Concentration (%) |
|
Emulsion stabilizer |
1 |
|
Tablet binder |
Up to 10 |
|
Thickener for lotions and cream |
Up to 2.5 |
Table 2. Pharmacopeial specifications for gaur gum
|
Test |
USP |
|
Appearance (powder) |
White to yellowish-white |
|
Viscosity (1% w/v dispersion) |
4860 cps |
|
Density |
1.492 g/cm3 |
|
Molecular weight |
≈ 220 000 |
|
pH (1 % w/v solution) |
5.5-7.5 |
|
Loss on drying |
≤ 15.0 % |
|
Acid-insoluble matter |
≤ 7.0 % |
|
Arsenic |
≤ 3 ppm |
|
Lead |
≤ 0.001 % |
|
Heavy metals |
≤ 0.002 % |
|
Protein |
≤ 10.0 % |
Table 3. Compositions of guar gum (Cyamopsis tetragonoloba seeds)
|
Compositon |
Concentration (%) |
|
Soluble fiber |
75 |
|
Insoluble fiber |
7.6 |
|
Crude protein |
2.16 |
|
Total lipid |
0.78 |
|
Moisture |
9.75 |
|
Ash |
0.5 |
References :
1.Kulkarni GT, Gowthamarajan K, Brahamajirao, Suresh B. Evaluation of binding properties of selected natural mucilages. J. Sci. Ind. Res. 2002, 61, 529-532.
2.Chaurasia MK, Jain SK. Polysaccharide for Colon Targeted Drug Delivery.Drug Deliv. 2004, 11, 129-148.
3. Chaurasia M, Chourasia MK , Jain NK, Jain A, Soni V, Gupta Y, Jain SK. Cross-Linked Guar Gum Microspheres: A Viable Approach for Improved Delivery of Anticancer Drugs for the Treatment of Colorectal Cancer. AAPS PharmSciTech. 2006, 7, 74.
4. Panariello G, Favaloro R, Forbicioni M, Caputo E, Barbucci R. Synthesis of a New Hydrogel, Based on Guar Gum, for Controlled Drug Release. Macromol. Symp. 2008, 266, 68–73.
5. Filbey JA, Charles SA. Shellac to PEGylation: 2500 year of the polymers in controlled release drug delivery. Drug Deliv. Tech. (2005), 5, 64-69.
6. Salyers AA, Vercellotti JR, West SHE, Wilkins TD. Fermentation of mucin and plant polysaccharides by strains of bacteroides from the human colon. Appl. Environ. Microbiol. 1977, 33, 319–322.
7. Krishnaiah YSR, Satyanarayan S, Rama Prasad YV. Studies of guar gum compression coated 5-aminosalicylic acid tablets for colon-specific drug delivery. Drug Dev. Ind. Pharm. 1999, 25, 651-657.
8. Krishnaiah YSR, Raju PV, Kumar BD, Bhaskar P, Satyanarayan V. Development of colon targeted drug delivery systems for mebendazole. J. Cont. Rel. 2001, 77, 87-95.
9. Feinstein W, Bartilucci AJ. Comparative study of selected disintegrant agents. J. Pharm Sci. 1966, 55, 332-334.
10. Sakr AM, Elsabbagh HM. Evaluation of gaur gum as atablet additive: a preliminary report. Pharm Ind. 1977, 39, 399-403.
11. Duru C, Colombo P, Gaudy D. A comparative study of the disintegrating efficiency of polysaccharides in a directly-tabletable formulation. Pharm. Tech. Int. 1992, 4, 15-16, 20, 22, 23.
12. Latchman L, Lieberman HA, Kanig, JL. The Theory and Practice of Industrial Pharmacy. 3rd Indian edition, Varghese Publishers, India, 1990, 592.
13. Goldstein AM, Alter EN, Seaman JK. Guar Gum. In: Whistler RL (Ed). Industrial Gums, Polysaccharides and their derivatives. Academic Press, New York, 1993, 303-321.
14. 15. 16. Gamal-Eldeen AM, Amer H, Helmy WA. Cancer chemopreventive and anti- inflammatory activities of chemically modified guar gum. Chem-Bio. Inter. 2006, 161, 229–240.
17. Frias AC, Sgarbier VC. Guar gum effects on food intake, blood serum lipids and glucose levels of Wistar rats. Plant Foods Hum. Nutr. 1998, 53, 15–28.
18. Bhandari U, Prasad DN. The effect of guar gum on serum and tissue cholesterol in experimentally hyperlipidemic rats. Ind. J. Pharmacol. 1991, 23, 268–270.
19. Goldstein AM, Alter EN, Seaman JK. Guar gum, in: R.L. Whistler (Ed.), Industrial Gums, Polysaccharides and their Derivatives, Academic Press, New York. 1973, 303–321.
20. Daas PJH, Schols HA, de Jongh HH. On the galactosyl distribution of commercial galactomannans. Carbohydr. Res. 2000, 329, 609–619.
21. FASEB (Federation of American Societies for Experimental Biology). Evaluation of the health aspects of guar gum as a food ingredient. Submitted under contract No. FDA (72-85). Bethesda. 1973, 1–12.
22. Strzelczyk AB, Ko?odziejska J, Zgoda MM. Application of guar gum biopolymer in the prescription of tablets with sodium ibuprofen – quality tests and pharmaceutical availability in vitro. Polim. Med. 2006, 36, 3-11.
23. Wellcome Foundation Ltd. Inhibition of gelation of polysaccharide gums in pharmaceuticals. Jap. Patent 55019290 A. 1980, 7.
24. Butt MS, Shahzadi N, Sharif MK, Nasir M. Guar gum: A miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Food sci. nutrit. 2007, 47, 389- 396. 25. Tiraferri A, Chen KL, Sethi R, Elimelech M. Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J. Colloid Interf. Sci. 2008, 324, 71–79.
26. Rowe R. Handbook of Pharmaceutical Excipients. Guar gum, Sixth edition, 2008, 315-317.
27. Lobenberg R. Smart materials: applications of nanotechnology in drug delivery and drug targeting. Proceedings of the International Conferences on MEMS, NANO and Smart Systems (ICMENS’03). 2003.
28. Sarmah JK, Bhattacharjee SK, Mahanta R, Mahanta R. Preparation of cross- linked guar gum nanospheres containing tamoxifen citrate by single step emulsion in situ polymer cross-linking method. J. Incl. Phenom. Macrocycl. Chem. DOI 10.1007/s10847-009-9589-7, 2009.
29. Goto T, Tanida N, Yoshinaga T, Sato S, Ball D, Wilding I, Kobayashi E, Fujimura A. Pharmaceutical design of a novel colon-targeted delivery system using two-layer-coated tablets of three different pharmaceutical formulations, supported by clinical evidence in humans. J. Cont. Rel. 2004, 97, 31-42.
30. Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Sci. 2003, 6, 33-66.
31. Al-Saidan, SM, Krishnaiah, YSR, Satyanarayana V, Bhaskar P, Karthikeyan, RS. Pharmacokinetic evaluation of guar gum based three-layer matrix tablets for oral controlled delivery of highly soluble metoprolol tartarate as a model drug. Eur. J. Pharm. Biopharm. 2004, 58, 697–703.
32. Sinha VR, Mittal BR, Kumria R. In vivo evaluation of time and site of disintegration of polysaccharide tablet prepared for colon-specific drug delivery. Int. J. Pharm. 2005, 289, 79–85.
33. Krishnaiah YS, Satyanarayana S, Rama Prasad YV, Narasimha Rao S. Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy human volunteers. J. Cont. Rel. 1998, 55, 245-52.
34. Al-Saidan S, Krishnaiah Y, Satyanarayana V, Rao G. In vitro and in vivo evaluation of guar gum-based matrix tablets of rofecoxib for colonic drug delivery. Curr. Drug Deliv. 2005, 2, 155-163.
35. Kenyon CJ, Nardi RV, Wong D, Hooper G, Wilding IR, Friend DR. Colonic delivery of dexamethasone: a pharmacoscintigraphic evaluation. Aliment. Pharmacol. Ther. 1997, 11, 205–213.
36. Sinha V, Mittal B, Kumria R. In vivo evaluation of time and site of disintegration of polysaccharide tablet prepared for colon specific drug delivery. Int. J. Pharm 2005, 289, 79-85.
37. Tugcu-Demiroez F, Acartuerk F, Takka S, Konus-Boyunaga O. In-vitro and in- vivo evaluation of mesalazine-guar gum matrix tablets for colonic drug delivery. J. Drug Target. 2004, 12, 105-112.
38. Krishnaiah YSR, Karthikeyan RS, Gouri Sankar V, Satyanarayana V. Three- layer guar gum matrix tablet formulations for oral controlled delivery of highly soluble trimetazidine dihydrochloride. J. Cont. Rel. 2002, 81, 45–56.
39. Krishnaiah YS, Karthikeyan RS, Satyanarayana V. A three-layer guar gum matrix tablet for oral controlled delivery of highly soluble metoprolol tartrate. Int. J. Pharm. 2002, 241, 353-66.
40. Narasimha Murthy S, Shobha Rani R, Hiremath, KLK. Evaluation of carboxymethyl guar films for the formulation of transdermal therapeutic systems. Int. J. Pharma. 2004, 272, 11–18.
41. Cunha PLR, Castro RR, Rocha FAC, de Paula RCM, Feitosa JPA. Low viscosity hydrogel of guar gum: Preparation and physicochemical characterization. Int. J. Biol. Macromol. 2005, 37, 99-104.
42. Chary RBR, Vani G, Rao YM. In Vitro and In Vivo Adhesion Testing of Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1999, 25, 685– 690.
43. Mathiowitz DE. Bioadhesive Drug Delivery Systems. Marcel Dakker. vol. 98. 1999, 551.
44. Deshmukh VN, Jadhav JK, Sakarkar DM. Formulation and in vitro evaluation of theophylline anhydrous bioadhesive tablets. Asian J. Pharma. 2009, 3, 54- 58.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

