About Authors:
Amit Jain*, Sandesh Jain, A.G. Hariharan, C.K. Sudhakar, Sanjay Jain
Department of Pharmaceutics,
Smriti College of pharmaceutical education, Indore-452001,
Madhya Pradesh, India
*amitjainpharma16@gmail.com
ABSTRACT
The present investigation deals with development of mucoadhesive thermosensitive pluronic lecithin organogel of clotrimazole for vaginal candidasis. To develop more effective treatment for vaginal candidasis, clotrimazole (CT) was formulated as pluronic lecithin organogels (PLOs). Several PLOs formulations composed of clotrimazole (1%) using the thermosensitive polymer Pluronic® F127, Soya lecithin with the mucoadhesive polymer Carbopol 934. Spreadibility, rheological behavior, drug content (%), mucoadhesive force, gelling capacity and in-vitro release profiles of the different formulations were determined. In vitro, sustained release of CT from PLOs was observed. In vivo antifungal activity of CT, tested against Candida albicans vaginitis in agar plate, was significantly prolonged after vaginal delivery using PLOs. These results indicate that CT-containing vaginal PLOs safe, convenient, and effective treatment of vaginal candidasis with reduced dosing interval.
Reference Id: PHARMATUTOR-ART-1297
INTRODUCTION
Genital tract infections, which are among the most frequent gynecological diseases[1]. It has been reported that 30–50% of vaginitis episodes are due to Candida infection and that two-thirds of all women experience acute episodes of vaginal candidasis at least once during their life time [2]. For the treatment of vaginitis, topical antifungal chemotherapy has been favored owing to the toxicity of antifungal drugs after systemic administration.
For vaginal delivery systems of antifungal agents to be more effective, they need to reside at the sitesof infection for a prolonged period. In additionconvenience of dosing methods is an important factor in the design of vaginal application forms. Of vaginal dosage forms, patients are known to better tolerate gels than inserts or ointments [3]. However, the direct application of gels onto the infected sites of the vagina might be difficult as well as inconvenient. Moreover, conventional gels do not remain for long at the site of application, leading to frequent dosing with antifungal agents.Vaginal candidasis, treated commonly with imidazole derivative antifungal agents such as clotrimazole since these drugs are locally active with no major side effects [4, 5].
Current vaginal delivery systems include creams, foams, gels, tablets, pessaries and irrigations, which are limited use because of less residence time at the genitourinary tract: they are removed rather rapidly by the self-cleansing action of the vaginal tract [6, 7]. Moreover, the physiological conditions imposed by the protective mechanism of the genital tract, limiting the residence time and thus impairing the therapeutic efficacy of the drug, make multiple and frequent administration necessary for treatment. Patient compliance when administering the dosage forms and following the repeated-dose therapeutic regimen is an important challenge in vaginal drug delivery. Patients are generally reported to tolerate organogels better than other dosage forms [8].
PLOs are clear, viscoelastic, biocompatible, and isotropic gels composed of phospholipids (lecithin), appropriate organic solvent, and a polar solvent[9,10].Ease of preparation and scale-up, easier quality monitoring, thermodynamic stability, enhanced topical performance along with biocompatibility, safety upon applications for prolonged period, and unique property, that it can incorporate both hydrophilic and lipophilic drugsmake the organogels a vehicle of choice for topical drug delivery.
Mucoadhesive vaginal organogels containing Carbopol enhancing the adhesion of the administered dosage form to the mucosal tissue have been added to thermosensitive organogels prepared with Pluronic f127[11-15]. Different techniques are adopted in drug delivery with thermosensitive organogels, including dispersing the drug in the organogel with a concentration higher than its solubility value and dispersing drug-loaded nanoparticles, liposomes, and drug: cyclodextrin complexes [16-20].
The objective of this study was to design a vaginal organogel formulation with thermosensitive and mucoadhesive properties to ensure longer residence at the infection site, providing a favorable release profile for the antifungal drug clotrimazole.
Materials and methods
Materials
CT was provided as gift sample by Apex Pharmaceuticals Pvt. Ltd. Ahmedabad. Pluronic F127 were supplied by Sigma chemicals and soya lecithin from Himedia laboratories (Mumbai). All other chemicals were of reagent grade and used further without purification.
Preparation of formulations
PLOs were prepared by using the cold method [20, 21]. Aqueous phase were prepared by dispersing Carbopol 934 in citrate-phosphate buffer (0.1 M, pH 4.0) at 4 ?C with gentle mixing. Pluronic F-127was then added to carbopol 934 solution and allowed to dissolve overnight at 4 ?C. Oil phase was prepared by dissolving soya lecithin and sorbic acid in appropriate quantity of isopropyl myristate. The mixture was kept overnight at 4ºC in refrigerator for complete dissolution of its constituents. CT was initially dissolved in the mixture of ethanol and polyethylene glycol (PEG) 400 (3:5), and mixed with lecithin isopropyl myristate solution., Finally aqueous phase (70%) was slowly added in oil phase (30%) in with stirring at 400 rpm using mechanical stirrer.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Determination of mucoadhesive force:
The mucoadhesive force of organogel onvaginal mucosal tissues was determined by means of mucoadhesive force measuring apparatus, fabricated in our laboratory.
Vaginal mucosal tissues were removed from Sprague-Dawley ratsand tissue were stored frozen in phosphate buffer at pH 5.5, and thawed to the room temperature before use. At the time of testing, a section of tissue was secured (keeping the mucosal side out) to the upper side of a glass vial using a cyanoacrylate adhesive. The diameter of each exposed mucosal membrane was1.5 cm. The vials were equilibrated and maintained at 37° for 10 min. One vial with a section of tissue was connected to the balance and the other vial was fixed on a height adjustable pan. To the exposed surface of the tissue attached on the vial, a constant amount of 0.1 g organogel was applied. Before applying the organogel, 150 μl of simulated vaginal fluid was evenly spread on the surface of the test membrane. The height of the vial was adjusted such that the organogel could adhere to the mucosal surface of both vials. Immediately, a constant force of 0.5 N (Newton) for 2 min was applied to ensure intimate contact between the tissue and the sample. The upper vial was then moved upwards at a constant force, while it was connected to the balance. Weights were added at a constant rate to the pan on the other side of the modified balance until the two vials were separated.The mucoadhesive force, expressed as the detachment stress in dynes/cm2, was determined from the minimal weights needed to detach the tissues from the surface of each formulation, using the following Eqn.[21, 22]
Detachment stress (dynes/cm2) = (m×g)/a,
Where ‘m’ is the weight added to the balance in grams; ‘g’ is the acceleration due to gravity taken as 980 cm/s2; and ‘a’ is the area of tissue exposed.
Effect of varying contact time (1, 2, 3, 5 and 10 min) was investigated for some of the organogel preparations to optimize initial contact time. In brief, formulations were allowed to be in contact with mucosa for carrying contact time (1, 2, 3, 5, and 10 min.), and the mucoadhesive force was determined as discussed above[23]. Contact time that resulted in maximum mucoadhesive strength was selected as optimum contact time required for adequate adhesion. All the above mentioned experiments were carried out in triplicates.
Measurement of viscosity of organogels:
Viscosity determinations of the prepared organogels were carried out by cone and plate geometry viscometer (Model: RV DV-E 230), using spindle No 7. Viscosity of organogels was measured at 10 rpmat a temperature of 25°C[24].The averages of three readings were used to calculate the viscosity. Evaluations were conducted in triplicate.
Spreadability:
For the determination of spreadability, excess of sample was applied between the two glass Slides and was compressed to uniform thickness by placing 1000 g weight for 5 min. Weight (50g) was added to the pan. The time required to separate the two slides, i.e. the time in which the upper glass slide moves over the lower plate was taken as measure of spreadability (S)[25]. S=M×L/T,
Where M = weight tide to upper slide, L = length moved on the glass slide, T = time taken.
Gelling capacity:
The gelling capacity was determined by placing a drop of the system in a vial containing 2 ml of simulated vaginal fluid (pH 5.5) freshly prepared and equilibrated at 37°Cand visually assessing the organogel formation and noting the time for gelation and thetime taken for the organogel formed to dissolve. Different grades were allotted as per the organogel integrity, weight and rate of formation of organogel with respect to time.
In vitro release studies:
The in vitro release of from different formulations was determined using a dialysis bag placed in a sealed glass vial under constant magnetic stirring. The gel formulations (2.5 g) were packed into the dialysis bags (Spectra/Por Cellulose EsterMembrane MWCO: 100 000 Da, Spectrum Labs, Rancho Dominguez, CA) sealed with closures of 50 mm (Spectrum Labs). The release medium was 100 mL 0.1M citrate-phosphate buffer (pH 5.5) containing 1% Tween 80, providing sink conditions for clotrimazole. The medium was maintained at 37ºC and stirred at 100 rpm. At various time intervals, 5 mL of dissolution fluid was collected. Levels of clotrimazole in the samples were analyzed with a Shimadzu UV-VIS 1760A spectrophotometer at λ = 262 nm. The exact amount of clotrimazole released from the formulation was calculated with a calibration curve with an analytically validated method (r2 =0.9958, repeatability coefficient of variation (CV) = 0.11%, reproducibility CV = 2.2%).
Antifungal efficacy studies:
The antifungal efficacy study against Candida albicans was determined by agar diffusion method employing ‘cup plate technique’. Sterile solutions of CT in DMSO (standard solution) and the developed organogel having the pH adjusted to 7.0 were poured into cups (0.1 ml of 0.1% w/v) bored into sterile malt yeast agar previously seeded with test organism. After allowing diffusion of the solutions for 2 h, the agar plates were incubated at 37°Cfor 48 h. The zone of inhibition (ZOI) was measured and compared with that of pure drug. The entire operation was carried out in aseptic condition throughout the study. Each solution was tested in triplicate. Both positive and negative controls were maintained throughout the study[26].
RESULTS AND DISCUSSION
The organogel formulations were optimized by factorial design method (using Design Expert software). Concentration of Soya lecithin, Pluronic F 127 and Carbopol 934, were three factors selected for optimization of formula.Compositions of various formulations used in the prepared PLOs are given in table 1.
TABLE 1: COMPOSITION OF VARIOUS FORMULATIONS
|
Formulation |
Clotrimazole (%) |
Soya Lecithin (%) |
Carbopol 934 (%) |
Pluronic F127 (%) |
Isopropyl myristate upto (ml) |
Sorbic acid (%) |
Water upto (ml) |
|
F1 |
1 |
2 |
0.8 |
50 |
100 |
0.2 |
100 |
|
F2 |
1 |
2 |
0.8 |
32.5 |
100 |
0.2 |
100 |
|
F3 |
1 |
2 |
0.2 |
15 |
100 |
0.2 |
100 |
|
F4 |
1 |
2 |
1.4 |
50 |
100 |
0.2 |
100 |
|
F5 |
1 |
4 |
1.4 |
50 |
100 |
0.2 |
100 |
|
F6 |
1 |
4 |
1.4 |
15 |
100 |
0.2 |
100 |
|
F7 |
1 |
4 |
0.2 |
15 |
100 |
0.2 |
100 |
|
F8 |
1 |
4 |
0.2 |
50 |
100 |
0.2 |
100 |
|
F9 |
1 |
6 |
0.8 |
50 |
100 |
0.2 |
100 |
|
F10 |
1 |
6 |
0.8 |
15 |
100 |
0.2 |
100 |
|
F11 |
1 |
6 |
0.2 |
32.5 |
100 |
0.2 |
100 |
|
F12 |
1 |
6 |
1.4 |
32.5 |
100 |
0.2 |
100 |
The prepared PLOs formulations were characterized on the basis of spreadibility, rheological behavior, drug content (%), mucoadhesive force, gelling capacity and in-vitro release profiles. The prepared PLOs formulations were white to pale yellow viscous creamy preparation with a smooth and homogeneous appearance. The pH values of all prepared formulation ranged from 5.5 to 6.5, which is considered acceptable to avoid the risk of irritation upon application to the skin as pH of skin 5.5.
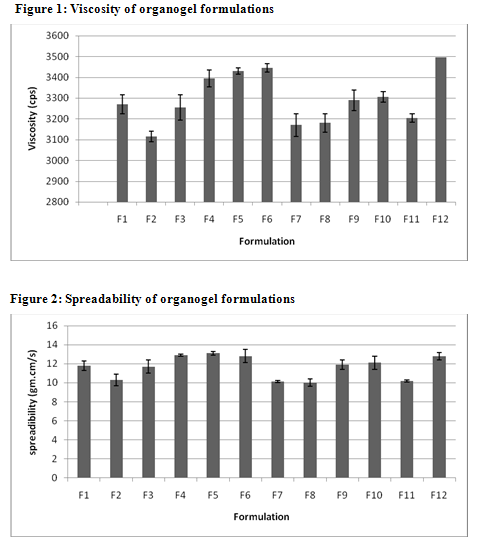
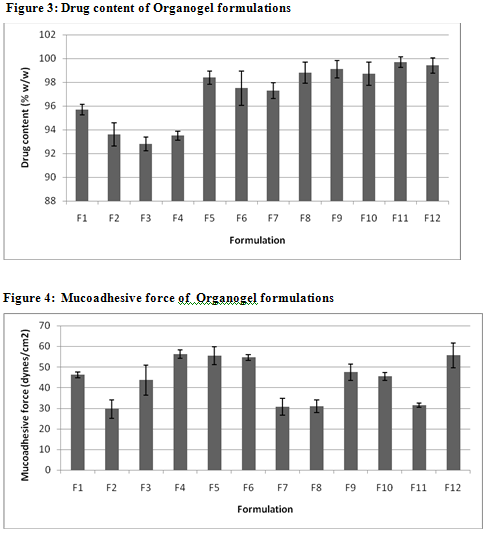
The values of spreadability indicate that the organogel is easily spreadable by small amount of shear. The spreadability of all formulations from 10.01 to 13.1. Viscosity determinations of the prepared organogels were carried out by cone and plate geometry viscometerusing spindle No 7. The highestviscosity was found in organogel contain high level of the Cabopol 934 concentration.
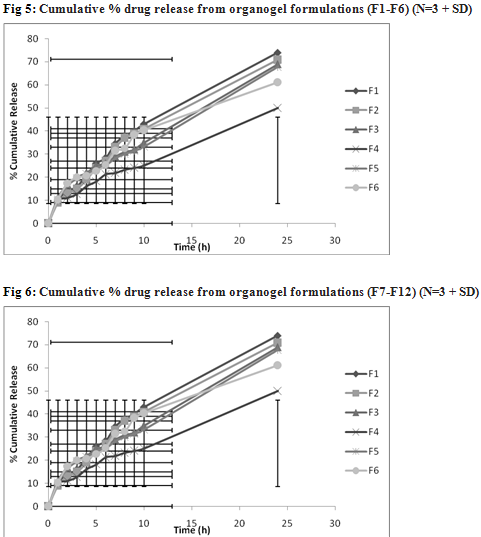
TABLE 2: CHARACTERISTICS OF VARIOUS CLOTRIMAZOLE ORGANOGEL FORMULATIONS
|
Formulations |
pH* |
Viscosity* (cps) |
Spreadbility (g.cm/s) |
Drug Content (%w/w) |
*Muco- adhesive force (dynes/cm2) |
Gelling Capacity |
|
F1 |
5.9 |
3270±25 |
11.8±0.6 |
95.7±0.33 |
46.2±2.4 |
++ |
|
F2 |
6.1 |
3115±45 |
10.3±0.7 |
93.6±1.44 |
29.6±1.09 |
+ |
|
F3 |
6.2 |
3255±20 |
11.7±0.4 |
92.8±2.45 |
43.6±3.4 |
++ |
|
F4 |
6.3 |
3395±45 |
12.9±0.4 |
93.5±0.44 |
56.3±5.3 |
+++ |
|
F5 |
5.8 |
3430±30 |
13.1±0.6 |
98.4±0.55 |
55.5±7.5 |
+++ |
|
F6 |
5.5 |
3445±55 |
12.8±0.1 |
97.5±0.99 |
54.6±5.6 |
+++ |
|
F7 |
5.8 |
3170±60 |
10.12±0.3 |
97.3±0.68 |
30.6±0.7 |
+ |
|
F8 |
6.2 |
3180±15 |
10.01±0.5 |
98.8±0.49 |
30.9±2.8 |
+ |
|
F9 |
6.1 |
3290±20 |
11.9±0.1 |
99.1±0.47 |
47.7±1.5 |
++ |
|
F10 |
6.4 |
3305±25 |
12.1±0.4 |
98.7±0.55 |
45.5±1.2 |
++ |
|
F11 |
6.2 |
3205±30 |
10.2±0.2 |
99.7±0.65 |
31.1±2.8 |
+ |
|
F12 |
6.5 |
3495±35 |
12.8±0.4 |
99.4±0.75 |
55.6±4.2 |
+++ |
*Average of three reading; + gel after few minutes, dissolved rapidly; ++gelation immediately, remains for few hours; +++ gelation immediately,
remains for extended period.
The drug content in organogel was found in range of 92.8 % to 99.7 %. The higher drug content found in F11 i.e. 99.7% due to the optimum concentration of pluronic F127 and soya lecithin. The in vitro release profiles of clotrimazole from its various organogel formulations are represented in Table 3. Higher drug release was observed with formulations F11. This finding may be due to presence of optimum level of carbopol 934 (1%) and soya lecithin (4%). Further increase in concentration of lecithin decreased cumulative % drug release which might be due to extensive formation of network like structure with very high viscosity.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
TABLE 3: Percentage Drug Release from Organogel Formulations (F1-F6)
|
Time (hr.) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
1 |
10±0.66 |
9±0.54 |
11±0.54 |
9.8±0.57 |
10.4±0.41 |
10.3±0.37 |
|
2 |
14±0.76 |
13±0.35 |
17±0.12 |
11.1±0.45 |
16.3±0.53 |
17.3±0.45 |
|
3 |
16±0.44 |
15±0.54 |
20±0.32 |
12.8±0.58 |
19.6±0.37 |
20±0.38 |
|
4 |
21±0.62 |
19±0.84 |
22±0.43 |
16.2±0.69 |
21.4±0.48 |
20.7±0.38 |
|
5 |
26±0.41 |
24±0.94 |
25±0.52 |
18.2±0.54 |
24±0.42 |
22.7±0.35 |
|
6 |
28.4±0.64 |
27±0.15 |
26±0.74 |
21.3±0.48 |
25.2±0.39 |
25.3±0.33 |
|
7 |
34.5±0.58 |
33±0.19 |
29±0.79 |
21.9±0.55 |
28.3±0.41 |
31.4±0.37 |
|
8 |
38±0.64 |
37±0.51 |
31±0.54 |
23.3±0.72 |
30.1±0.50 |
32.5±0.39 |
|
9 |
40±0.76 |
39±0.23 |
32±0.83 |
24.12±0.70 |
31.5±0.34 |
38.4±0.43 |
|
10 |
43±0.85 |
41±0.11 |
35±0.19 |
25.13±0.89 |
33.3±0.43 |
40.4±0.39 |
|
24 |
74±0.69 |
71±0.78 |
69±0.45 |
50.06±0.35 |
67.9±0.43 |
61.14±0.46 |
TABLE 4: Percentage Drug Release from Organogel Formulations (F6-F12)
|
Time (hr.) |
F7 |
F8 |
F9 |
F10 |
F11 |
F12 |
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
1 |
9.83±0.29 |
8.76±0.37 |
9.5±0.34 |
10.3±0.31 |
25.6±0.42 |
15.5±0.73 |
|
2 |
10.71±0.45 |
10.88±0.38 |
16±0.51 |
17.3±0.39 |
31.4±0.49 |
19±0.38 |
|
3 |
13.1±0.31 |
12.21±0.38 |
17±0.59 |
20±0.18 |
36.4±0.51 |
24.8±0.34 |
|
4 |
14.1±0.40 |
15.09±0.34 |
23±0.73 |
25.4±0.89 |
37.1±0.59 |
29.7±0.25 |
|
5 |
15.4±0.46 |
20±0.35 |
26±0.83 |
28.4±0.82 |
38.2±0.29 |
32.2±0.12 |
|
6 |
16.4±0.42 |
22.74±0.45 |
29±0.61 |
31±0.64 |
40.7±0.74 |
34.3±0.67 |
|
7 |
17.72±0.29 |
25.9±0.44 |
32±0.90 |
35±0.53 |
41.7±0.78 |
37.9±0.64 |
|
8 |
19.03±0.38 |
31.5±0.49 |
35±0.41 |
38±0.42 |
43.5±0.81 |
40.3±0.59 |
|
9 |
21.52±0.47 |
34.22±0.34 |
43±0.71 |
44±0.17 |
51.68±0.89 |
46.6±0.49 |
|
10 |
23.65±0.39 |
36.48±0.35 |
45±0.42 |
47±0.83 |
54.51±0.39 |
49.6±0.43 |
|
24 |
41.06±0.36 |
60.7±0.41 |
77±0.49 |
81±0.19 |
93.8±0.29 |
86±0.11 |
MIC ranges (mg/mL) was 0.5−16. No intrazonal growth was observed in the antifungal method. Inhibition zone diameters ranged from 5 to 35mm . Interclass correlation coefficients (ICCs) and 95% confidence intervals for comparing the methods were calculated using log2 transformed data.
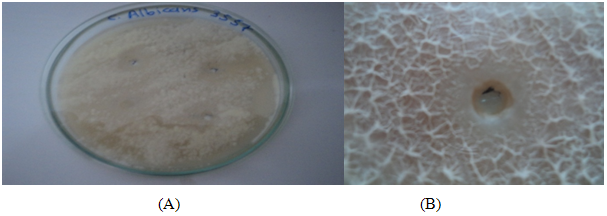
Fig 7: Antifungal efficacy study against Candida albicansby agar diffusion method employing cup plate technique A: Front view B: Zoom section
CONCLUSION
The data in this study support the potential effectiveness of a vaginal gel with mucoadhesive properties to ensure longer residence time in the application site because of prolonged release properties Controlled release of the incorporated drug is achieved, suggesting better patient compliance and higher therapeutic efficacy.
ACKNOWLEDGEMENTS The authors are thankful to Smriti College of pharmaceutical Education, Indore for providing facilities to carry out this work.
REFERENCE:
1. J.Wang, Bacterial vaginosis, Prim. Care Update Ob/Gyns. 7 (2000) 181–185
2. R.A. Ross, M.T. Lee, A.B. Onderdonk, Effect of Candida albicans infection and clotrimazole treatment on vaginal microflora, in vitro, Obstet. Gynaecol. 86 (1995) 925–953
3. K. Edsman, J. Carlfors, R. Petersson, Rheological evaluation of poloxamer as an in situ gel for ophthalmic use, Eur. J. Pharm. Sci. 6 (1998) 105–112.
4. Ritter W, Patzschke K, Krause U, Stettendorf S. Pharmacokinetic fundamentals of vaginal treatment with clotrimazole. Chemotherapy (1982)28-43
5. Sawyer PR, Brogden RN, Pinder RM, Speight TM, Avery GS. Clotrimazole: a review of its antifungal activity and therapeutic efficacy. Drugs. (1975);9:424Y447.
6. Deshpande AA, Rhodes CT, Danish M. Intravaginal drug delivery.Drug Dev Ind Pharm. (1992);18:1225Y1279.
7. Wolfsson AD. Intravaginal drug delivery technologies. In: Rathbone M, ed. Modified-Release Drug Delivery Technology. New York, NY: Marcel Dekker;( 2002):759Y774.
8. Hardy E, Jimenes AL, de Padua KS, Zanewald LJD. Women’s preferences for vaginal antimicrobial contraceptives, III: choice of a formulation, applicator and packaging. Contraception. (1998); 58:245Y249.
9. Scartazzini R, Luisi PL. Organogels from lecithins. J Phys Chem. (1988);92:829Y833.
10. Schurtenberger P, Scartazzini R, Magid LJ, Leser ME, Luisi PL. Structural and dynamic properties of polymer-like reverse micelles. J Phys Chem. (1990); 94:3695Y3701
11. Ceschel GC, Maffei P, Lombardi Borgia S, Ronchi C, Rossi S. Development of a mucoadhesive dosage form for vaginal administration. Drug Dev Ind Pharm. (2001);27:541Y547.
12. Chang JY, Oh YK, Choi H, Kim YB, Kim CK. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. (2002);241:155Y163
13. Chang JY, Oh YK, Kong HS, et al. Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels in vaginitis. J Control Release. (2002); 82:39Y50.
14. Chu JS, Amidon GL, Weiner ND, Goldberg AH. Mixture experimental design in the development of a mucoadhesive gel formulation. Pharm Res. (1991);8:1401Y1407.
15. Park H, Robinson JR. Physicochemical properties of water insoluble polymers important to mucin/epithelial adhesion. J Control Release.(1985); 2:47Y57.
16. Veyries ML, Couarrazze G, Geiger S, et al. Controlled release of vancomycin from poloxamer 407 gels. Int J Pharm. (1999);192:183Y193.
17. Desai SD, Blanchard J. Pluronic F127-based ocular delivery system containing biodegradable polyisobutylcyanoacrylate nanocapsules of pilocarpine. Drug Deliv. (2000);7:201Y207.
18. Bochot A, Fattal E, Grossiord JL, Puisieux F, Couvreur P. Characterization of a new ocular delivery system based on a dispersion of liposomes in a thermosensitive gel. Int J Pharm. (1998);162:119Y127.
19. Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-β-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. (2002); 233:159Y167
20. Kim EY, Complexation in poloxamer gel for ophthalmic delivery. Int J Pharm. (2003); 33:150Y147.
21. H.G. Choi, J.H. Jung, J.M. Ryu, S.J. Yoon, Y.K. Oh, C.K. Kim, Development of in situ gelling and mucoadhesiveacetaminophen liquid suppository, Int. J. Pharm. 16 (1998) 533–544.
22. M. Morishita, J.M. Barichello, K. Takayama, Y. Chiba, S. Tokiwa, T. Nagai, Pluronic F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin, Int. J. Pharm. 212 (2001) 289–293.
23.Wu C, Qi H, Chen W, Huang C, Su C, Li W, et al. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi (2007);127:183-91.
24. Ikanth PS, Mishra B. Floating in situ gelling system for stomach site- specific delivery of clarithromycin to eradicate H. pylori. J Control Release (2008);125:33-41.
25. El laithy HM, El-Shaboury KM. The development of cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPS PharmSciTech (2002);3:77-85.
26. Mitra J, Mohammad RR, Hedayte S. Mucoadhesive and drug release properties of benzocaine gel. Iranian J Pharm Sci (2006);2:185-94.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











.png)

